Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety
- PMID: 20590588
- PMCID: PMC2935998
- DOI: 10.1111/j.1476-5381.2010.00709.x
Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety
Abstract
Background and purpose: The major drug-metabolizing enzymes for the oxidation of oxycodone are CYP2D6 and CYP3A. A high interindividual variability in the activity of these enzymes because of genetic polymorphisms and/or drug-drug interactions is well established. The possible role of an active metabolite in the pharmacodynamics of oxycodone has been questioned and the importance of CYP3A-mediated effects on the pharmacokinetics and pharmacodynamics of oxycodone has been poorly explored.
Experimental approach: We conducted a randomized crossover (five arms) double-blind placebo-controlled study in 10 healthy volunteers genotyped for CYP2D6. Oral oxycodone (0.2 mg x kg(-1)) was given alone or after inhibition of CYP2D6 (with quinidine) and/or of CYP3A (with ketoconazole). Experimental pain (cold pressor test, electrical stimulation, thermode), pupil size, psychomotor effects and toxicity were assessed.
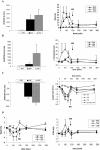
Key results: CYP2D6 activity was correlated with oxycodone experimental pain assessment. CYP2D6 ultra-rapid metabolizers experienced increased pharmacodynamic effects, whereas cold pressor test and pupil size were unchanged in CYP2D6 poor metabolizers, relative to extensive metabolizers. CYP2D6 blockade reduced subjective pain threshold (SPT) for oxycodone by 30% and the response was similar to placebo. CYP3A4 blockade had a major effect on all pharmacodynamic assessments and SPT increased by 15%. Oxymorphone C(max) was correlated with SPT assessment (rho(S)= 0.7) and the only independent positive predictor of SPT. Side-effects were observed after CYP3A4 blockade and/or in CYP2D6 ultra-rapid metabolizers.
Conclusions and implications: The modulation of CYP2D6 and CYP3A activities had clear effects on oxycodone pharmacodynamics and these effects were dependent on CYP2D6 genetic polymorphism.
Figures


Similar articles
-
The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone.Br J Pharmacol. 2010 Jun;160(4):907-18. doi: 10.1111/j.1476-5381.2010.00673.x. Br J Pharmacol. 2010. PMID: 20590587 Free PMC article. Clinical Trial.
-
Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone.Eur J Clin Pharmacol. 2011 Jan;67(1):63-71. doi: 10.1007/s00228-010-0893-3. Epub 2010 Sep 21. Eur J Clin Pharmacol. 2011. PMID: 20857093 Clinical Trial.
-
The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism.Basic Clin Pharmacol Toxicol. 2009 Apr;104(4):335-44. doi: 10.1111/j.1742-7843.2009.00378.x. Epub 2009 Feb 27. Basic Clin Pharmacol Toxicol. 2009. PMID: 19281600 Clinical Trial.
-
Pharmacogenomics of oxycodone: a narrative literature review.Pharmacogenomics. 2021 Apr;22(5):275-290. doi: 10.2217/pgs-2020-0143. Epub 2021 Mar 17. Pharmacogenomics. 2021. PMID: 33728947 Free PMC article. Review.
-
Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications.Drugs. 2013 May;73(6):533-43. doi: 10.1007/s40265-013-0036-0. Drugs. 2013. PMID: 23605691 Review.
Cited by
-
Sensitive UHPLC-MS/MS quantification method for 4β- and 4α-hydroxycholesterol in plasma for accurate CYP3A phenotyping.J Lipid Res. 2022 Mar;63(3):100184. doi: 10.1016/j.jlr.2022.100184. Epub 2022 Feb 16. J Lipid Res. 2022. PMID: 35181316 Free PMC article.
-
Super analgesia of intrathecal morphine may be related to ABCB1 (MDR1) gene polymorphism.J Pain Res. 2018 Jul 20;11:1355-1357. doi: 10.2147/JPR.S156919. eCollection 2018. J Pain Res. 2018. PMID: 30050319 Free PMC article.
-
Pharmacoeconomics of genotyping-based treatment decisions in patients with chronic pain.Pain Rep. 2017 Aug 11;2(5):e615. doi: 10.1097/PR9.0000000000000615. eCollection 2017 Sep. Pain Rep. 2017. PMID: 29392230 Free PMC article.
-
Genetic Testing for Opioid Pain Management: A Primer.Pain Ther. 2017 Jun;6(1):93-105. doi: 10.1007/s40122-017-0069-2. Epub 2017 Apr 13. Pain Ther. 2017. PMID: 28409480 Free PMC article.
-
Effects of Short-Term Oxycodone Maintenance on Experimental Pain Responses in Physically Dependent Opioid Abusers.J Pain. 2017 Jul;18(7):825-834. doi: 10.1016/j.jpain.2017.02.433. Epub 2017 Mar 6. J Pain. 2017. PMID: 28274698 Free PMC article.
References
-
- Backlund M, Lindgren L, Kajimoto Y, Rosenberg PH. Comparison of epidural morphine and oxycodone for pain after abdominal surgery. J Clin Anesth. 1997;9:30–35. - PubMed
-
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:163–172. - PubMed
-
- Caraco Y, Sheller J, Wood AJ. Pharmacogenetic determinants of codeine induction by rifampin: the impact on codeine's respiratory, psychomotor and miotic effects. J Pharmacol Exp Ther. 1997;281:330–336. - PubMed
-
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48:2165–2171. - PubMed
-
- Childers SR, Creese I, Snowman AM, Synder SH. Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol. 1979;55:11–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

