Influence of 5-aminosalicylic acid on 6-thioguanosine phosphate metabolite levels: a prospective study in patients under steady thiopurine therapy
- PMID: 20590602
- PMCID: PMC2936018
- DOI: 10.1111/j.1476-5381.2010.00731.x
Influence of 5-aminosalicylic acid on 6-thioguanosine phosphate metabolite levels: a prospective study in patients under steady thiopurine therapy
Abstract
Background and purpose: 5-aminosalicylate (5-ASA) raises levels of 6-thioguanine nucleotides (6-TGN), the active metabolites of thiopurines such as azathioprine (AZA). Changes in levels of each individual TGN - 6-thioguanosine mono-, di- and triphosphate (6-TGMP, 6-TGDP, 6-TGTP) - and of 6-methylmercaptopurine ribonucleotides (6-MMPR) after 5-ASA are not known.
Experimental approach: Effects of increasing 5-ASA doses on AZA metabolites were investigated prospectively in 22 patients with inflammatory bowel disease in 4-week study periods. Patients started with 2 g 5-ASA daily, and then were increased to 4 g daily and followed by a washout period. Thiopurine doses remained unchanged throughout the entire study. Levels of 6-TGMP, 6-TGDP, 6-TGTP and 6-MMPR as well as of 5-ASA and N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA) were determined each study period.
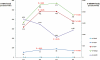
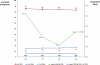
Key results: Median baseline levels in 17 patients of 6-TGDP, 6-TGTP and 6-MMPR were 52, 319 and 1676 pmol per 8 x 10(8) red blood cells respectively. After co-administration of 2 g 5-ASA daily, median 6-TGDP and 6-TGTP levels increased but median 6-MMPR levels were unchanged. Increasing 5-ASA to 4 g daily did not affect median 6-TGDP and 6-TGTP levels, but median 6-MMPR levels decreased. After discontinuation of 5-ASA, both 6-TGDP and 6-TGTP levels decreased and median 6-MMPR levels increased. The 6-TGTP/(6-TGDP+6-TGTP)-ratio did not change during the study, but 6-MMPR/6-TGN ratios decreased.
Conclusions and implications: Individual 6-TGN metabolites increased after addition of 5-ASA, but 6-MMPR-levels and the 6-MMPR/6-TGN ratios decreased. Further studies are needed to decide whether this pharmacokinetic interaction would result in improvement of efficacy and/or increased risk of toxicity of AZA.
Figures

References
-
- Allgayer H, Ahnfelt NO, Kruis W, Klotz U, Frank-Holmberg K, Soderberg HN, et al. Colonic N-acetylation of 5-aminosalicylic acid in inflammatory bowel disease. Gastroenterology. 1989;97:38–41. - PubMed
-
- Ansari A, Elliott T, Baburajan B, Mayhead P, O'Donohue J, Chocair P, et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:734–741. - PubMed
-
- de Boer NK, van Bodegraven AA, Jharap B, De Graaf P, Mulder CJ. Drug insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007a;4:686–694. - PubMed
-
- de Boer NK, Wong DR, Jharap B, De Graaf P, Hooymans PM, Mulder CJ, et al. Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol. 2007b;102:2747–2753. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

