Differences in the expression of endogenous efflux transporters in MDR1-transfected versus wildtype cell lines affect P-glycoprotein mediated drug transport
- PMID: 20590635
- PMCID: PMC2938816
- DOI: 10.1111/j.1476-5381.2010.00801.x
Differences in the expression of endogenous efflux transporters in MDR1-transfected versus wildtype cell lines affect P-glycoprotein mediated drug transport
Abstract
Background and purpose: P-glycoprotein (Pgp) efflux assays are widely used to identify Pgp substrates. The kidney cell lines Madin-Darby canine kidney (MDCK)-II and LLC-PK1, transfected with human MDR1 (ABCB1) are used to provide recombinant models of drug transport. Endogenous transporters in these cells may contribute to the activities of recombinant transporters, so that drug transport in MDR1-transfected cells is often corrected for the transport obtained in parental (wildtype) cells. However, expression of endogenous transporters may vary between transfected and wildtype cells, so that this correction may cause erroneous data. Here, we have measured the expression of endogenous efflux transporters in transfected and wildtype MDCK-II or LLC cells and the consequences for Pgp-mediated drug transport.
Experimental approach: Using quantitative real-time RT-PCR, we determined the expression of endogenous Mdr1 mRNA and other efflux transporters in wildtype and MDR1-transfected MDCK-II and LLC cells. Transcellular transport was measured with the test substrate vinblastine.
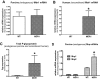
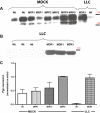
Key results: In MDR1-transfected MDCK cells, expression of endogenous (canine) Mdr1 and Mrp2 (Abcc2) mRNA was markedly lower than in wildtype cells, whereas MDR1-transfected LLC cells exhibited comparable Mdr1 but strikingly higher Mrp2 mRNA levels than wildtype cells. As a consequence, transport of vinblastine by human Pgp in efflux experiments was markedly underestimated when transport in MDR1-transfected MDCK cells was corrected for transport obtained in wildtype cells. This problem did not occur in LLC cells.
Conclusions and implications: Differences in the expression of endogenous efflux transporters between transfected and wildtype MDCK cells provide a potential bias for in vitro studies on Pgp-mediated drug transport.
Figures



Similar articles
-
Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT).Drug Metab Dispos. 2002 Apr;30(4):457-63. doi: 10.1124/dmd.30.4.457. Drug Metab Dispos. 2002. PMID: 11901101
-
Investigation of MDR1-overexpressing cell lines to derive a quantitative prediction approach for brain disposition using in vitro efflux activities.Eur J Pharm Sci. 2020 Jan 15;142:105119. doi: 10.1016/j.ejps.2019.105119. Epub 2019 Nov 1. Eur J Pharm Sci. 2020. PMID: 31682973
-
Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man.Biochem Pharmacol. 2002 Dec 1;64(11):1569-78. doi: 10.1016/s0006-2952(02)01355-2. Biochem Pharmacol. 2002. PMID: 12429346
-
Mechanism of multidrug recognition by MDR1/ABCB1.Cancer Sci. 2007 Sep;98(9):1303-10. doi: 10.1111/j.1349-7006.2007.00538.x. Epub 2007 Jun 30. Cancer Sci. 2007. PMID: 17608770 Free PMC article. Review.
-
A Novel Approach to Refractory Epilepsy by Targeting Pgp Peripherally and Centrally: Therapeutic Targets and Future Perspectives.CNS Neurol Disord Drug Targets. 2020;19(10):741-749. doi: 10.2174/1871527319999200819093109. CNS Neurol Disord Drug Targets. 2020. PMID: 32814543 Review.
Cited by
-
Generation of Knockout and Transgenic Zebrafish to Characterize Abcc4 Functions in Detoxification and Efflux of Lead.Int J Mol Sci. 2021 Feb 19;22(4):2054. doi: 10.3390/ijms22042054. Int J Mol Sci. 2021. PMID: 33669601 Free PMC article.
-
Role of Human Breast Cancer Related Protein versus P-Glycoprotein as an Efflux Transporter for Benzylpenicillin: Potential Importance at the Blood-Brain Barrier.PLoS One. 2016 Jun 14;11(6):e0157576. doi: 10.1371/journal.pone.0157576. eCollection 2016. PLoS One. 2016. PMID: 27300692 Free PMC article.
-
Genomic Knockout of Endogenous Canine P-Glycoprotein in Wild-Type, Human P-Glycoprotein and Human BCRP Transfected MDCKII Cell Lines by Zinc Finger Nucleases.Pharm Res. 2015 Jun;32(6):2060-71. doi: 10.1007/s11095-014-1599-5. Epub 2014 Dec 19. Pharm Res. 2015. PMID: 25522789
-
Disruption of small molecule transporter systems by Transporter-Interfering Chemicals (TICs).FEBS Lett. 2020 Dec;594(23):4158-4185. doi: 10.1002/1873-3468.14005. Epub 2020 Dec 9. FEBS Lett. 2020. PMID: 33222203 Free PMC article. Review.
-
A face-to-face comparison of claudin-5 transduced human brain endothelial (hCMEC/D3) cells with porcine brain endothelial cells as blood-brain barrier models for drug transport studies.Fluids Barriers CNS. 2020 Aug 26;17(1):53. doi: 10.1186/s12987-020-00212-5. Fluids Barriers CNS. 2020. PMID: 32843059 Free PMC article.
References
-
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. - PubMed
-
- Artursson P. Epithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990;79:476–482. - PubMed
-
- Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, Löscher W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007a;52:333–346. - PubMed
-
- Baltes S, Fedrowitz M, Luna-Tortós C, Potschka H, Löscher W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007b;320:331–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

