Differential effects of thin and thick filament disruption on zebrafish smooth muscle regulatory proteins
- PMID: 20591105
- PMCID: PMC3902778
- DOI: 10.1111/j.1365-2982.2010.01545.x
Differential effects of thin and thick filament disruption on zebrafish smooth muscle regulatory proteins
Abstract
Background: The smooth muscle actin binding proteins Caldesmon and Tropomyosin (Tm) promote thin filament assembly by stabilizing actin polymerization, however, whether filament assembly affects either the stability or activation of these and other smooth muscle regulatory proteins is not known.
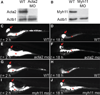
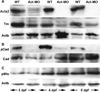
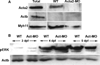
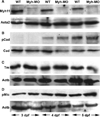
Methods: Measurement of smooth muscle regulatory protein levels in wild type zebrafish larvae following antisense knockdown of smooth muscle actin (Acta2) and myosin heavy chain (Myh11) proteins, and in colourless mutants that lack enteric nerves. Comparison of intestinal peristalsis in wild type and colourless larvae.
Key results: Knockdown of Acta2 led to reduced levels of phospho-Caldesmon and Tm. Total Caldesmon and phospho-myosin light chain (p-Mlc) levels were unaffected. Knockdown of Myh11 had no effect on the levels of either of these proteins. Phospho-Caldesmon and p-Mlc levels were markedly reduced in colourless mutants that have intestinal motility comparable with wild type larvae.
Conclusions & inferences: These in vivo findings provide new information regarding the activation and stability of smooth muscle regulatory proteins in zebrafish larvae and their role in intestinal peristalsis in this model organism.
Figures

Similar articles
-
Smooth muscle caldesmon modulates peristalsis in the wild type and non-innervated zebrafish intestine.Neurogastroenterol Motil. 2012 Mar;24(3):288-99. doi: 10.1111/j.1365-2982.2011.01844.x. Neurogastroenterol Motil. 2012. PMID: 22316291 Free PMC article.
-
Role of thin-filament regulatory proteins in relaxation of colonic smooth muscle contraction.Am J Physiol Gastrointest Liver Physiol. 2009 Nov;297(5):G958-66. doi: 10.1152/ajpgi.00201.2009. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 20501443 Free PMC article.
-
Over expression of smooth muscle thin filament associated proteins in the bladder wall of diabetics.J Urol. 2005 Jul;174(1):360-4. doi: 10.1097/01.ju.0000161602.18671.c7. J Urol. 2005. PMID: 15947690
-
[Caldesmon and calponin are proteins, participating in the regulation of myosin and actin interaction in nonmuscle and smooth muscle cells].Biokhimiia. 1991 Aug;56(8):1347-68. Biokhimiia. 1991. PMID: 1782263 Review. Russian.
-
Structural interactions between actin, tropomyosin, caldesmon and calcium binding protein and the regulation of smooth muscle thin filaments.Acta Physiol Scand. 1998 Dec;164(4):401-14. doi: 10.1111/j.1365-201x.1998.tb10696.x. Acta Physiol Scand. 1998. PMID: 9887964 Review.
Cited by
-
The enteric nervous system promotes intestinal health by constraining microbiota composition.PLoS Biol. 2017 Feb 16;15(2):e2000689. doi: 10.1371/journal.pbio.2000689. eCollection 2017 Feb. PLoS Biol. 2017. PMID: 28207737 Free PMC article.
-
Ultra-structural identification of interstitial cells of Cajal in the zebrafish Danio rerio.Cell Tissue Res. 2012 Aug;349(2):483-91. doi: 10.1007/s00441-012-1434-4. Epub 2012 May 25. Cell Tissue Res. 2012. PMID: 22628160 Free PMC article.
-
Development of the zebrafish enteric nervous system.Methods Cell Biol. 2011;101:143-60. doi: 10.1016/B978-0-12-387036-0.00006-2. Methods Cell Biol. 2011. PMID: 21550442 Free PMC article. Review.
-
Smooth muscle tension induces invasive remodeling of the zebrafish intestine.PLoS Biol. 2012;10(9):e1001386. doi: 10.1371/journal.pbio.1001386. Epub 2012 Sep 4. PLoS Biol. 2012. PMID: 22973180 Free PMC article.
-
Graded effects of unregulated smooth muscle myosin on intestinal architecture, intestinal motility and vascular function in zebrafish.Dis Model Mech. 2016 May 1;9(5):529-40. doi: 10.1242/dmm.023309. Epub 2016 Feb 18. Dis Model Mech. 2016. PMID: 26893369 Free PMC article.
References
-
- Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. - PubMed
-
- Ansari S, Alahyan M, Marston SB, El-Mezgueldi M. Role of caldesmon in the Ca2+ regulation of smooth muscle thin filaments: evidence for a cooperative switching mechanism. J Biol Chem. 2008;283:47–56. - PubMed
-
- Kordowska J, Huang R, Wang CL. Phosphorylation of caldesmon during smooth muscle contraction and cell migration or proliferation. J Biomed Sci. 2006;13:159–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

