Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis
- PMID: 20591166
- PMCID: PMC2906448
- DOI: 10.1186/1465-9921-11-90
Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis
Abstract
Background: Viral bronchiolitis is the leading cause of hospitalization in young infants. It is associated with the development of childhood asthma and contributes to morbidity and mortality in the elderly. Currently no therapies effectively attenuate inflammation during the acute viral infection, or prevent the risk of post-viral asthma. We hypothesized that early treatment of a paramyxoviral bronchiolitis with azithromycin would attenuate acute and chronic airway inflammation.
Methods: Mice were inoculated with parainfluenza type 1, Sendai Virus (SeV), and treated daily with PBS or azithromycin for 7 days post-inoculation. On day 8 and 21 we assessed airway inflammation in lung tissue, and quantified immune cells and inflammatory mediators in bronchoalveolar lavage (BAL).
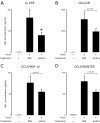
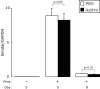
Results: Compared to treatment with PBS, azithromycin significantly attenuated post-viral weight loss. During the peak of acute inflammation (day 8), azithromycin decreased total leukocyte accumulation in the lung tissue and BAL, with the largest fold-reduction in BAL neutrophils. This decreased inflammation was independent of changes in viral load. Azithromycin significantly attenuated the concentration of BAL inflammatory mediators and enhanced resolution of chronic airway inflammation evident by decreased BAL inflammatory mediators on day 21.
Conclusions: In this mouse model of paramyxoviral bronchiolitis, azithromycin attenuated acute and chronic airway inflammation. These findings demonstrate anti-inflammatory effects of azithromycin that are not related to anti-viral activity. Our findings support the rationale for future prospective randomized clinical trials that will evaluate the effects of macrolides on acute viral bronchiolitis and their long-term consequences.
Figures




References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. - PubMed
-
- Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am J Respir Crit Care Med. 2001;163(3 Pt 2):S2–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

