Automated quantitative live cell fluorescence microscopy
- PMID: 20591990
- PMCID: PMC2908775
- DOI: 10.1101/cshperspect.a000455
Automated quantitative live cell fluorescence microscopy
Abstract
Advances in microscopy automation and image analysis have given biologists the tools to attempt large scale systems-level experiments on biological systems using microscope image readout. Fluorescence microscopy has become a standard tool for assaying gene function in RNAi knockdown screens and protein localization studies in eukaryotic systems. Similar high throughput studies can be attempted in prokaryotes, though the difficulties surrounding work at the diffraction limit pose challenges, and targeting essential genes in a high throughput way can be difficult. Here we will discuss efforts to make live-cell fluorescent microscopy based experiments using genetically encoded fluorescent reporters an automated, high throughput, and quantitative endeavor amenable to systems-level experiments in bacteria. We emphasize a quantitative data reduction approach, using simulation to help develop biologically relevant cell measurements that completely characterize the cell image. We give an example of how this type of data can be directly exploited by statistical learning algorithms to discover functional pathways.
Figures


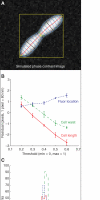
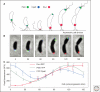
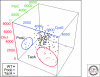

Similar articles
-
High-throughput RNAi screening by time-lapse imaging of live human cells.Nat Methods. 2006 May;3(5):385-90. doi: 10.1038/nmeth876. Nat Methods. 2006. PMID: 16628209
-
Quantitative fluorescence microscopy and image deconvolution.Methods Cell Biol. 2013;114:407-26. doi: 10.1016/B978-0-12-407761-4.00017-8. Methods Cell Biol. 2013. PMID: 23931516
-
Digital autofocus methods for automated microscopy.Methods Enzymol. 2006;414:620-32. doi: 10.1016/S0076-6879(06)14032-X. Methods Enzymol. 2006. PMID: 17110214 Review.
-
An image score inference system for RNAi genome-wide screening based on fuzzy mixture regression modeling.J Biomed Inform. 2009 Feb;42(1):32-40. doi: 10.1016/j.jbi.2008.04.007. Epub 2008 Apr 29. J Biomed Inform. 2009. PMID: 18547870 Free PMC article.
-
Quantitative time-lapse fluorescence microscopy in single cells.Annu Rev Cell Dev Biol. 2009;25:301-27. doi: 10.1146/annurev.cellbio.042308.113408. Annu Rev Cell Dev Biol. 2009. PMID: 19575655 Free PMC article. Review.
Cited by
-
Mining high-throughput experimental data to link gene and function.Trends Biotechnol. 2011 Apr;29(4):174-82. doi: 10.1016/j.tibtech.2011.01.001. Trends Biotechnol. 2011. PMID: 21310501 Free PMC article. Review.
-
Image analysis in fluorescence microscopy: bacterial dynamics as a case study.Bioessays. 2012 May;34(5):427-36. doi: 10.1002/bies.201100148. Epub 2012 Mar 13. Bioessays. 2012. PMID: 22415868 Free PMC article. Review.
-
Immunomodulatory mechanisms of lactobacilli.Microb Cell Fact. 2011 Aug 30;10 Suppl 1(Suppl 1):S17. doi: 10.1186/1475-2859-10-S1-S17. Epub 2011 Aug 30. Microb Cell Fact. 2011. PMID: 21995674 Free PMC article.
-
Uncovering the mechanism for polar sequestration of the major bacterial sugar regulator by high-throughput screens and 3D interaction modeling.Cell Rep. 2025 Mar 25;44(3):115436. doi: 10.1016/j.celrep.2025.115436. Epub 2025 Mar 17. Cell Rep. 2025. PMID: 40100851 Free PMC article.
-
Analysis of gene expression levels in individual bacterial cells without image segmentation.Biochem Biophys Res Commun. 2012 May 11;421(3):425-30. doi: 10.1016/j.bbrc.2012.03.117. Epub 2012 Apr 1. Biochem Biophys Res Commun. 2012. PMID: 22487793 Free PMC article.
References
-
- Biondi EG, Skerker JM, Arif M, Prasol MS, Perchuk BS, Laub MT 2006. A phosphorelay system controls stalk biogenesis during cell cycle progression in Caulobacter crescentus. Mol Microbiol 59: 386–401 - PubMed
-
- Boland MV, Murphy RF 1999. Automated analysis of patterns in fluorescence-microscope images. Trends Cell Biol 9: 201–202 - PubMed
-
- Boland MV, Murphy RF 2001. A neural network classifier capable of recognizing the patterns of all major subcellular structures in fluorescence microscope images of HeLa cells. Bioinformatics 17: 1213–1223 - PubMed
-
- Boland MV, Markey MK, Murphy RF 1998. Automated recognition of patterns characteristic of subcellular structures in fluorescence microscopy images. Cytometry 33: 366–375 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
