Two atypical L-cysteine-regulated NADPH-dependent oxidoreductases involved in redox maintenance, L-cystine and iron reduction, and metronidazole activation in the enteric protozoan Entamoeba histolytica
- PMID: 20592025
- PMCID: PMC2930688
- DOI: 10.1074/jbc.M110.106310
Two atypical L-cysteine-regulated NADPH-dependent oxidoreductases involved in redox maintenance, L-cystine and iron reduction, and metronidazole activation in the enteric protozoan Entamoeba histolytica
Abstract
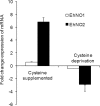
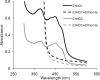
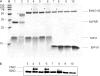
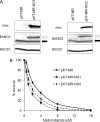
We discovered novel catalytic activities of two atypical NADPH-dependent oxidoreductases (EhNO1/2) from the enteric protozoan parasite Entamoeba histolytica. EhNO1/2 were previously annotated as the small subunit of glutamate synthase (glutamine:2-oxoglutarate amidotransferase) based on similarity to authentic bacterial homologs. As E. histolytica lacks the large subunit of glutamate synthase, EhNO1/2 were presumed to play an unknown role other than glutamine/glutamate conversion. Transcriptomic and quantitative reverse PCR analyses revealed that supplementation or deprivation of extracellular L-cysteine caused dramatic up- or down-regulation, respectively, of EhNO2, but not EhNO1 expression. Biochemical analysis showed that these FAD- and 2[4Fe-4S]-containing enzymes do not act as glutamate synthases, a conclusion which was supported by phylogenetic analyses. Rather, they catalyze the NADPH-dependent reduction of oxygen to hydrogen peroxide and L-cystine to L-cysteine and also function as ferric and ferredoxin-NADP(+) reductases. EhNO1/2 showed notable differences in substrate specificity and catalytic efficiency; EhNO1 had lower K(m) and higher k(cat)/K(m) values for ferric ion and ferredoxin than EhNO2, whereas EhNO2 preferred L-cystine as a substrate. In accordance with these properties, only EhNO1 was observed to physically interact with intrinsic ferredoxin. Interestingly, EhNO1/2 also reduced metronidazole, and E. histolytica transformants overexpressing either of these proteins were more sensitive to metronidazole, suggesting that EhNO1/2 are targets of this anti-amebic drug. To date, this is the first report to demonstrate that small subunit-like proteins of glutamate synthase could play an important role in redox maintenance, L-cysteine/L-cystine homeostasis, iron reduction, and the activation of metronidazole.
Figures

References
-
- Rosenbaum K., Jahnke K., Curti B., Hagen W. R., Schnackerz K. D., Vanoni M. A. (1998) Biochemistry 37, 17598–17609 - PubMed
-
- Tóth A., Takács M., Groma G., Rákhely G., Kovács K. L. (2008) FEMS Microbiol. Lett. 282, 8–14 - PubMed
-
- Stutz H. E., Reid S. J. (2004) Biochim. Biophys. Acta 1676, 71–82 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

