Imaging cell wall architecture in single Zinnia elegans tracheary elements
- PMID: 20592039
- PMCID: PMC2938135
- DOI: 10.1104/pp.110.155242
Imaging cell wall architecture in single Zinnia elegans tracheary elements
Abstract
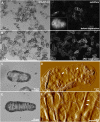
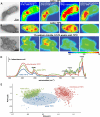
The chemical and structural organization of the plant cell wall was examined in Zinnia elegans tracheary elements (TEs), which specialize by developing prominent secondary wall thickenings underlying the primary wall during xylogenesis in vitro. Three imaging platforms were used in conjunction with chemical extraction of wall components to investigate the composition and structure of single Zinnia TEs. Using fluorescence microscopy with a green fluorescent protein-tagged Clostridium thermocellum family 3 carbohydrate-binding module specific for crystalline cellulose, we found that cellulose accessibility and binding in TEs increased significantly following an acidified chlorite treatment. Examination of chemical composition by synchrotron radiation-based Fourier-transform infrared spectromicroscopy indicated a loss of lignin and a modest loss of other polysaccharides in treated TEs. Atomic force microscopy was used to extensively characterize the topography of cell wall surfaces in TEs, revealing an outer granular matrix covering the underlying meshwork of cellulose fibrils. The internal organization of TEs was determined using secondary wall fragments generated by sonication. Atomic force microscopy revealed that the resulting rings, spirals, and reticulate structures were composed of fibrils arranged in parallel. Based on these combined results, we generated an architectural model of Zinnia TEs composed of three layers: an outermost granular layer, a middle primary wall composed of a meshwork of cellulose fibrils, and inner secondary wall thickenings containing parallel cellulose fibrils. In addition to insights in plant biology, studies using Zinnia TEs could prove especially productive in assessing cell wall responses to enzymatic and microbial degradation, thus aiding current efforts in lignocellulosic biofuel production.
Figures




References
-
- Barceló AR. (1995) Peroxidase and not laccase is the enzyme responsible for cell-wall lignification in the secondary thickening of xylem vessels in Lupinus. Protoplasma 186: 41–44
-
- Benova-Kakosova A, Digonnet C, Goubet F, Ranocha P, Jauneau A, Pesquet E, Barbier O, Zhang Z, Capek P, Dupree P, et al. (2006) Galactoglucomannans increase cell population density and alter the protoxylem/metaxylem tracheary element ratio in xylogenic cultures of Zinnia. Plant Physiol 142: 696–709 - PMC - PubMed
-
- Bierhorst DW. (1960) Observations on tracheary elements. Phytomorphology 10: 249–305
-
- Burgess J, Linstead P. (1984) In-vitro tracheary element formation: structural studies and the effect of tri-iodobenzoic acid. Planta 160: 481–489 - PubMed

