Moderate influence of human APOBEC3F on HIV-1 replication in primary lymphocytes
- PMID: 20592068
- PMCID: PMC2937628
- DOI: 10.1128/JVI.02630-09
Moderate influence of human APOBEC3F on HIV-1 replication in primary lymphocytes
Abstract
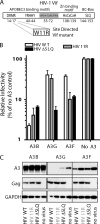
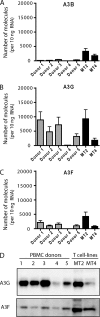
Multiple APOBEC3 proteins are expressed in HIV-1 target cells, but their individual contributions to viral suppression when expressed at endogenous levels remain largely unknown. We used an HIV NL4-3 mutant that selectively counteracts APOBEC3G (A3G) but not APOBEC3F (A3F) to dissect the relative contribution of A3F to the inhibition of HIV-1 replication in primary human lymphocytes (peripheral blood mononuclear cells [PBMCs]). This HIV Vif mutant replicated similarly to wild-type virus in PBMCs, suggesting that the effect of A3F on HIV restriction in these cells is limited. The different A3F variants found in PMBC donors displayed either comparable activity or less activity than wild-type A3F. Lastly, the endogenous A3F mRNA and protein expression levels in PBMCs were considerably lower than those of A3G. Our results suggest that A3F neutralization is dispensable for HIV-1 replication in primary human T-lymphocytes.
Figures

Similar articles
-
APOBEC3G restricts HIV-1 to a greater extent than APOBEC3F and APOBEC3DE in human primary CD4+ T cells and macrophages.J Virol. 2013 Jan;87(1):444-53. doi: 10.1128/JVI.00676-12. Epub 2012 Oct 24. J Virol. 2013. PMID: 23097438 Free PMC article.
-
Mechanism of Enhanced HIV Restriction by Virion Coencapsidated Cytidine Deaminases APOBEC3F and APOBEC3G.J Virol. 2017 Jan 18;91(3):e02230-16. doi: 10.1128/JVI.02230-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881650 Free PMC article.
-
APOBEC3G and APOBEC3F Act in Concert To Extinguish HIV-1 Replication.J Virol. 2016 Apr 14;90(9):4681-4695. doi: 10.1128/JVI.03275-15. Print 2016 May. J Virol. 2016. PMID: 26912618 Free PMC article.
-
Multiple APOBEC3 restriction factors for HIV-1 and one Vif to rule them all.J Mol Biol. 2014 Mar 20;426(6):1220-45. doi: 10.1016/j.jmb.2013.10.033. Epub 2013 Nov 2. J Mol Biol. 2014. PMID: 24189052 Free PMC article. Review.
-
Evasion of adaptive immunity by HIV through the action of host APOBEC3G/F enzymes.AIDS Res Ther. 2017 Sep 12;14(1):44. doi: 10.1186/s12981-017-0173-8. AIDS Res Ther. 2017. PMID: 28893290 Free PMC article. Review.
Cited by
-
IFN-α treatment inhibits acute Friend retrovirus replication primarily through the antiviral effector molecule Apobec3.J Immunol. 2013 Feb 15;190(4):1583-90. doi: 10.4049/jimmunol.1202920. Epub 2013 Jan 11. J Immunol. 2013. PMID: 23315078 Free PMC article.
-
The activity spectrum of Vif from multiple HIV-1 subtypes against APOBEC3G, APOBEC3F, and APOBEC3H.J Virol. 2012 Jan;86(1):49-59. doi: 10.1128/JVI.06082-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013041 Free PMC article.
-
APOBEC3G polymorphism as a selective barrier to cross-species transmission and emergence of pathogenic SIV and AIDS in a primate host.PLoS Pathog. 2013;9(10):e1003641. doi: 10.1371/journal.ppat.1003641. Epub 2013 Oct 3. PLoS Pathog. 2013. PMID: 24098115 Free PMC article.
-
Role of APOBEC3F Gene Variation in HIV-1 Disease Progression and Pneumocystis Pneumonia.PLoS Genet. 2016 Mar 4;12(3):e1005921. doi: 10.1371/journal.pgen.1005921. eCollection 2016 Mar. PLoS Genet. 2016. PMID: 26942578 Free PMC article.
-
Different mutagenic potential of HIV-1 restriction factors APOBEC3G and APOBEC3F is determined by distinct single-stranded DNA scanning mechanisms.PLoS Pathog. 2014 Mar 20;10(3):e1004024. doi: 10.1371/journal.ppat.1004024. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24651717 Free PMC article.
References
-
- Doehle, B. P., A. Schafer, and B. R. Cullen. 2005. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology 339:281-288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

