Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms
- PMID: 20592069
- PMCID: PMC2937635
- DOI: 10.1128/JVI.01069-10
Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms
Abstract
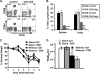
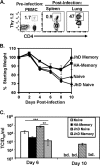
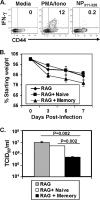
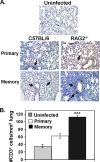
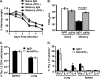
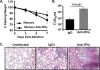
Memory CD4 T cells specific for influenza virus are generated from natural infection and vaccination, persist long-term, and recognize determinants in seasonal and pandemic influenza virus strains. However, the protective potential of these long-lived influenza virus-specific memory CD4 T cells is not clear, including whether CD4 T-cell helper or effector functions are important in secondary antiviral responses. Here we demonstrate that memory CD4 T cells specific for H1N1 influenza virus directed protective responses to influenza virus challenge through intrinsic effector mechanisms, resulting in enhanced viral clearance, recovery from sublethal infection, and full protection from lethal challenge. Mice with influenza virus hemagglutinin (HA)-specific memory CD4 T cells or polyclonal influenza virus-specific memory CD4 T cells exhibited protection from influenza virus challenge that occurred in the presence of CD8-depleting antibodies in B-cell-deficient mice and when CD4 T cells were transferred into lymphocyte-deficient RAG2(-/-) mice. Moreover, the presence of memory CD4 T cells mobilized enhanced T-cell recruitment and immune responses in the lung. Neutralization of gamma interferon (IFN-gamma) production in vivo abrogated memory CD4 T-cell-mediated protection from influenza virus challenge by HA-specific memory T cells and heterosubtypic protection by polyclonal memory CD4 T cells. Our results indicate that memory CD4 T cells can direct enhanced protection from influenza virus infection through mobilization of immune effectors in the lung, independent of their helper functions. These findings have important implications for the generation of universal influenza vaccines by promoting long-lived protective CD4 T-cell responses.
Figures

References
-
- Allan, W., Z. Tabi, A. Cleary, and P. C. Doherty. 1990. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J. Immunol. 144:3980-3986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

