Telomerase trafficking and assembly in Xenopus oocytes
- PMID: 20592184
- PMCID: PMC2894659
- DOI: 10.1242/jcs.063750
Telomerase trafficking and assembly in Xenopus oocytes
Abstract
The core components of telomerase are telomerase RNA (TR) and telomerase reverse transcriptase (TERT). In vertebrate cells, TR and TERT have been reported to associate with intranuclear structures, including Cajal bodies and nucleoli as well as telomeres. Here, we examined the time course of both TR localization and assembly of TR with TERT in Xenopus oocytes. The major trafficking pathway for microinjected TR is through Cajal bodies into the nucleoplasm, with a fraction of TR found in nucleoli at later time points. Telomerase assembly precedes nucleolar localization of TR, and TR mutants that do not localize to nucleoli form active enzyme, indicating that localization of TR to nucleoli is not required for assembly with TERT. Assembly of telomerase coincides with Cajal-body localization; however, assembly is also unaffected by a CAB-box mutation (which significantly reduces association with Cajal bodies), suggesting that Cajal-body localization is not important for assembly. Our results suggest that assembly of TR with TERT occurs in the nucleoplasm. Unexpectedly, however, our experiments reveal that disruption of the CAB box does not eliminate early targeting to Cajal bodies, indicating that a role for Cajal bodies in telomerase assembly cannot be excluded on the basis of existing knowledge.
Figures

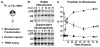

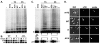
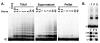

References
-
- Aubert G., Lansdorp P. M. (2008). Telomeres and aging. Physiol. Rev. 88, 557-579 - PubMed
-
- Autexier C., Lue N. F. (2006). The structure and function of telomerase reverse transcriptase. Annu. Rev. Biochem. 75, 493-517 - PubMed
-
- Beattie T. L., Zhou W., Robinson M. O., Harrington L. (1998). Reconstitution of human telomerase activity in vitro. Curr. Biol. 8, 177-180 - PubMed
-
- Blasco M. A. (2007). Telomere length, stem cells and aging. Nat. Chem. Biol. 3, 640-649 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

