Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1
- PMID: 20592197
- PMCID: PMC2923804
- DOI: 10.1523/JNEUROSCI.5229-09.2010
Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1
Abstract
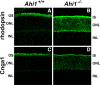
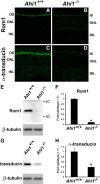
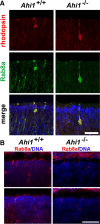
Vertebrate photoreceptors have a modified cilium composed of a basal body, axoneme and outer segment. The outer segment includes stacked membrane discs, containing opsin and the signal transduction apparatus mediating phototransduction. In photoreceptors, two distinct classes of vesicles are trafficked. Synaptic vesicles are transported down the axon to the synapse, whereas opsin-containing vesicles are transported to the outer segment. The continuous replacement of the outer segments imposes a significant biosynthetic and trafficking burden on the photoreceptors. Here, we show that Ahi1, a gene that when mutated results in the neurodevelopmental disorder, Joubert syndrome (JBTS), is required for photoreceptor sensory cilia formation and the development of photoreceptor outer segments. In mice with a targeted deletion of Ahi1, photoreceptors undergo early degeneration. Whereas synaptic proteins are correctly trafficked, photoreceptor outer segment proteins fail to be transported appropriately or are significantly reduced in their expression levels (i.e., transducin and Rom1) in Ahi1(-/-) mice. We show that vesicular targeting defects in Ahi1(-/-) mice are cilium specific, and our evidence suggests that the defects are caused by a decrease in expression of the small GTPase Rab8a, a protein required for accurate polarized vesicular trafficking. Thus, our results suggest that Ahi1 plays a role in stabilizing the outer segment proteins, transducin and Rom1, and that Ahi1 is an important component of Rab8a-mediated vesicular trafficking in photoreceptors. The retinal degeneration observed in Ahi1(-/-) mice recapitulates aspects of the retinal phenotype observed in patients with JBTS and suggests the importance of Ahi1 in photoreceptor function.
Figures

Similar articles
-
Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking.Hum Mol Genet. 2009 Oct 15;18(20):3926-41. doi: 10.1093/hmg/ddp335. Epub 2009 Jul 22. Hum Mol Genet. 2009. PMID: 19625297 Free PMC article.
-
ARL13B, a Joubert Syndrome-Associated Protein, Is Critical for Retinogenesis and Elaboration of Mouse Photoreceptor Outer Segments.J Neurosci. 2019 Feb 20;39(8):1347-1364. doi: 10.1523/JNEUROSCI.1761-18.2018. Epub 2018 Dec 20. J Neurosci. 2019. PMID: 30573647 Free PMC article.
-
AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis.Nat Genet. 2010 Feb;42(2):175-80. doi: 10.1038/ng.519. Epub 2010 Jan 17. Nat Genet. 2010. PMID: 20081859 Free PMC article.
-
Protein networks and complexes in photoreceptor cilia.Subcell Biochem. 2007;43:209-35. doi: 10.1007/978-1-4020-5943-8_10. Subcell Biochem. 2007. PMID: 17953396 Review.
-
Photoreceptor outer segment as a sink for membrane proteins: hypothesis and implications in retinal ciliopathies.Hum Mol Genet. 2017 Aug 1;26(R1):R75-R82. doi: 10.1093/hmg/ddx163. Hum Mol Genet. 2017. PMID: 28453661 Free PMC article. Review.
Cited by
-
Review of Ocular Manifestations of Joubert Syndrome.Genes (Basel). 2018 Dec 4;9(12):605. doi: 10.3390/genes9120605. Genes (Basel). 2018. PMID: 30518138 Free PMC article. Review.
-
Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies.Int J Mol Sci. 2022 Apr 28;23(9):4883. doi: 10.3390/ijms23094883. Int J Mol Sci. 2022. PMID: 35563274 Free PMC article. Review.
-
The Biology of Ciliary Dynamics.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a027904. doi: 10.1101/cshperspect.a027904. Cold Spring Harb Perspect Biol. 2017. PMID: 28062565 Free PMC article. Review.
-
Small GTPases Rab8a and Rab11a Are Dispensable for Rhodopsin Transport in Mouse Photoreceptors.PLoS One. 2016 Aug 16;11(8):e0161236. doi: 10.1371/journal.pone.0161236. eCollection 2016. PLoS One. 2016. PMID: 27529348 Free PMC article.
-
Loss of Ahi1 affects early development by impairing BM88/Cend1-mediated neuronal differentiation.J Neurosci. 2013 May 8;33(19):8172-84. doi: 10.1523/JNEUROSCI.0119-13.2013. J Neurosci. 2013. PMID: 23658157 Free PMC article.
References
-
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. - PubMed
-
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annual review of genomics and human genetics. 2006;7:125–148. - PubMed
-
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–1184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
