Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome
- PMID: 20592249
- PMCID: PMC2974588
- DOI: 10.1182/blood-2010-03-276972
Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome
Abstract
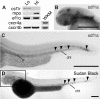
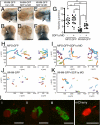
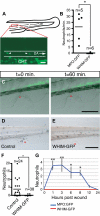
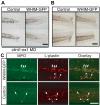
CXCR4 is a G protein-coupled chemokine receptor that has been implicated in the pathogenesis of primary immunodeficiency disorders and cancer. Autosomal dominant gain-of-function truncations of CXCR4 are associated with warts, hypo-gammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, a primary immunodeficiency disorder characterized by neutropenia and recurrent infections. Recent progress has implicated CXCR4-SDF1 (stromal cell-derived factor 1) signaling in regulating neutrophil homeostasis, but the precise role of CXCR4-SDF1 interactions in regulating neutrophil motility in vivo is not known. Here, we use the optical transparency of zebrafish to visualize neutrophil trafficking in vivo in a zebrafish model of WHIM syndrome. We demonstrate that expression of WHIM mutations in zebrafish neutrophils induces neutrophil retention in hematopoietic tissue, impairing neutrophil motility and wound recruitment. The neutrophil retention signal induced by WHIM truncation mutations is SDF1 dependent, because depletion of SDF1 with the use of morpholino oligonucleotides restores neutrophil chemotaxis to wounds. Moreover, localized activation of a genetically encoded, photoactivatable Rac guanosine triphosphatase is sufficient to direct migration of neutrophils that express the WHIM mutation. The findings suggest that this transgenic zebrafish model of WHIM syndrome may provide a valuable tool to screen for agents that modify CXCR4-SDF1 retention signals.
Figures


Comment in
-
A WHIM-sical zebrafish.Blood. 2010 Oct 14;116(15):2621-2. doi: 10.1182/blood-2010-07-296426. Blood. 2010. PMID: 20947686 Free PMC article.
References
-
- Doitsidou M, Reichman-Fried M, Stebler J, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111(5):647–659. - PubMed
-
- Alkhatib G, Berger EA. HIV coreceptors: from discovery and designation to new paradigms and promise. Eur J Med Res. 2007;12(9):375–384. - PubMed
-
- Gelmini S, Mangoni M, Serio M, Romagnani P, Lazzeri E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest. 2008;31(9):809–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

