Adverse events associated with testosterone administration
- PMID: 20592293
- PMCID: PMC3440621
- DOI: 10.1056/NEJMoa1000485
Adverse events associated with testosterone administration
Abstract
Background: Testosterone supplementation has been shown to increase muscle mass and strength in healthy older men. The safety and efficacy of testosterone treatment in older men who have limitations in mobility have not been studied.
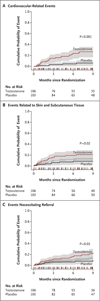
Methods: Community-dwelling men, 65 years of age or older, with limitations in mobility and a total serum testosterone level of 100 to 350 ng per deciliter (3.5 to 12.1 nmol per liter) or a free serum testosterone level of less than 50 pg per milliliter (173 pmol per liter) were randomly assigned to receive placebo gel or testosterone gel, to be applied daily for 6 months. Adverse events were categorized with the use of the Medical Dictionary for Regulatory Activities classification. The data and safety monitoring board recommended that the trial be discontinued early because there was a significantly higher rate of adverse cardiovascular events in the testosterone group than in the placebo group.
Results: A total of 209 men (mean age, 74 years) were enrolled at the time the trial was terminated. At baseline, there was a high prevalence of hypertension, diabetes, hyperlipidemia, and obesity among the participants. During the course of the study, the testosterone group had higher rates of cardiac, respiratory, and dermatologic events than did the placebo group. A total of 23 subjects in the testosterone group, as compared with 5 in the placebo group, had cardiovascular-related adverse events. The relative risk of a cardiovascular-related adverse event remained constant throughout the 6-month treatment period. As compared with the placebo group, the testosterone group had significantly greater improvements in leg-press and chest-press strength and in stair climbing while carrying a load.
Conclusions: In this population of older men with limitations in mobility and a high prevalence of chronic disease, the application of a testosterone gel was associated with an increased risk of cardiovascular adverse events. The small size of the trial and the unique population prevent broader inferences from being made about the safety of testosterone therapy. (ClinicalTrials.gov number, NCT00240981.)
2010 Massachusetts Medical Society
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Testosterone deficiency and replacement in older men.N Engl J Med. 2010 Jul 8;363(2):189-91. doi: 10.1056/NEJMe1006197. N Engl J Med. 2010. PMID: 20647215 No abstract available.
-
Reproductive endocrinology: Testosterone-replacement therapy in older men with limited mobility: is it safe?Nat Rev Endocrinol. 2010 Sep;6(9):471. doi: 10.1038/nrendo.2010.108. Nat Rev Endocrinol. 2010. PMID: 20803797 No abstract available.
-
Adverse events associated with testosterone administration.N Engl J Med. 2010 Nov 4;363(19):1866; author reply 1866-7. doi: 10.1056/NEJMc1009326. N Engl J Med. 2010. PMID: 21047233 No abstract available.
-
Adverse events associated with testosterone administration.N Engl J Med. 2010 Nov 4;363(19):1866; author reply 1866-7. doi: 10.1056/NEJMc1009326. N Engl J Med. 2010. PMID: 21047234 No abstract available.
-
Adverse events associated with testosterone administration.N Engl J Med. 2010 Nov 4;363(19):1865-6; author reply 1866-7. doi: 10.1056/NEJMc1009326. N Engl J Med. 2010. PMID: 21047235 No abstract available.
-
Adverse events associated with testosterone administration.N Engl J Med. 2010 Nov 4;363(19):1865; author reply 1866-7. doi: 10.1056/NEJMc1009326. N Engl J Med. 2010. PMID: 21047236 No abstract available.
-
ACP Journal Club. Testosterone increased risk for adverse events in older men with mobility limitations.Ann Intern Med. 2010 Dec 21;153(12):JC6-7. doi: 10.7326/0003-4819-153-12-201012210-02007. Ann Intern Med. 2010. PMID: 21173408 No abstract available.
-
Words of wisdom. Re: Adverse events associated with testosterone administration.Eur Urol. 2011 Mar;59(3):465. doi: 10.1016/j.eururo.2010.12.027. Eur Urol. 2011. PMID: 21414881 No abstract available.
References
-
- Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. - PubMed
-
- von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E. Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology. 2006;52:359–365. - PubMed
-
- Metter EJ, Schrager M, Ferrucci L, Talbot LA. Evaluation of movement speed and reaction time as predictors of all-cause mortality in men. J Gerontol A Biol Sci Med Sci. 2005;60:840–846. - PubMed
-
- Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R. The prevalence of functional limitations and disability in older persons in the US: data from the National Health and Nutrition Examination Survey III. J Am Geriatr Soc. 2000;48:1132–1135. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
