hNaa10p contributes to tumorigenesis by facilitating DNMT1-mediated tumor suppressor gene silencing
- PMID: 20592467
- PMCID: PMC2912195
- DOI: 10.1172/JCI42275
hNaa10p contributes to tumorigenesis by facilitating DNMT1-mediated tumor suppressor gene silencing
Abstract
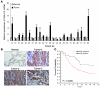
Hypermethylation-mediated tumor suppressor gene silencing plays a crucial role in tumorigenesis. Understanding its underlying mechanism is essential for cancer treatment. Previous studies on human N-alpha-acetyltransferase 10, NatA catalytic subunit (hNaa10p; also known as human arrest-defective 1 [hARD1]), have generated conflicting results with regard to its role in tumorigenesis. Here we provide multiple lines of evidence indicating that it is oncogenic. We have shown that hNaa10p overexpression correlated with poor survival of human lung cancer patients. In vitro, enforced expression of hNaa10p was sufficient to cause cellular transformation, and siRNA-mediated depletion of hNaa10p impaired cancer cell proliferation in colony assays and xenograft studies. The oncogenic potential of hNaa10p depended on its interaction with DNA methyltransferase 1 (DNMT1). Mechanistically, hNaa10p positively regulated DNMT1 enzymatic activity by facilitating its binding to DNA in vitro and its recruitment to promoters of tumor suppressor genes, such as E-cadherin, in vivo. Consistent with this, interaction between hNaa10p and DNMT1 was required for E-cadherin silencing through promoter CpG methylation, and E-cadherin repression contributed to the oncogenic effects of hNaa10p. Together, our data not only establish hNaa10p as an oncoprotein, but also reveal that it contributes to oncogenesis through modulation of DNMT1 function.
Figures


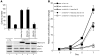


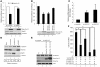
References
-
- Issa JP, et al. Increased cytosine DNA-methyltransferase activity during colon cancer progression. . J Natl Cancer Inst. 1993;85(15):1235–1240. - PubMed
-
- Vertino PM, Issa JP, Pereira-Smith OM, Baylin SB. Stabilization of DNA methyltransferase levels and CpG island hypermethylation precede SV40-induced immortalization of human fibroblasts. Cell Growth Differ. 1994;5(12):1395–1402. - PubMed
-
- Belinsky SA, Nikula KJ, Baylin SB, Issa JP. A microassay for measuring cytosine DNA methyltransferase activity during tumor progression. Toxicol Lett. 1995;82–83:335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

