Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels
- PMID: 20592471
- PMCID: PMC2912188
- DOI: 10.1172/JCI41985
Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels
Abstract
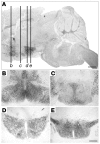
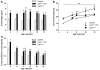
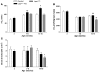
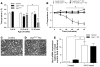
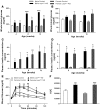
Distinct populations of leptin-sensing neurons in the hypothalamus, midbrain, and brainstem contribute to the regulation of energy homeostasis. To assess the requirement for leptin signaling in the hypothalamus, we crossed mice with a floxed leptin receptor allele (Leprfl) to mice transgenic for Nkx2.1-Cre, which drives Cre expression in the hypothalamus and not in more caudal brain regions, generating LeprNkx2.1KO mice. From weaning, LeprNkx2.1KO mice exhibited phenotypes similar to those observed in mice with global loss of leptin signaling (Leprdb/db mice), including increased weight gain and adiposity, hyperphagia, cold intolerance, and insulin resistance. However, after 8 weeks of age, LeprNkx2.1KO mice maintained stable adiposity levels, whereas the body fat percentage of Leprdb/db animals continued to escalate. The divergence in the adiposity phenotypes of Leprdb/db and LeprNkx2.1KO mice with age was concomitant with increased rates of linear growth and energy expenditure in LeprNkx2.1KO mice. These data suggest that remaining leptin signals in LeprNkx2.1KO mice mediate physiological adaptations that prevent the escalation of the adiposity phenotype in adult mice. The persistence of severe adiposity in LeprNkx2.1KO mice, however, suggests that compensatory actions of circuits regulating growth and energy expenditure are not sufficient to reverse obesity established at an early age.
Figures

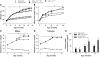
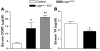
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

