Involvement of nitric oxide in a rat model of carrageenin-induced pleurisy
- PMID: 20592757
- PMCID: PMC2879545
- DOI: 10.1155/2010/682879
Involvement of nitric oxide in a rat model of carrageenin-induced pleurisy
Abstract
Some evidence indicates that nitric oxide (NO) contributes to inflammation, while other evidence supports the opposite conclusion. To clarify the role of NO in inflammation, we studied carrageenin-induced pleurisy in rats treated with an NO donor (NOC-18), a substrate for NO formation (L-arginine), and/or an NO synthase inhibitor (S-(2-aminoethyl) isothiourea or N(G)-nitro-L-arginine). We assessed inflammatory cell migration, nitrite/nitrate values, lipid peroxidation and pro-inflammatory mediators. NOC-18 and L-arginine reduced the migration of inflammatory cells and edema, lowered oxidative stress, and normalized antioxidant enzyme activities. NO synthase inhibitors increased the exudate formation and inflammatory cell number, contributed to oxidative stress, induced an oxidant/antioxidant imbalance by maintaining high O(2) (-), and enhanced the production of pro-inflammatory mediators. L-arginine and NOC-18 reversed the proinflammatory effects of NO synthase inhibitors, perhaps by reducing the expression of adhesion molecules on endothelial cells. Thus, our results indicate that NO is involved in blunting-not enhancing-the inflammatory response.
Figures
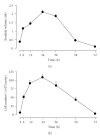
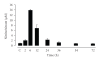

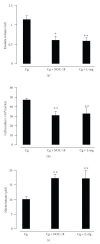

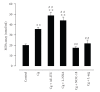

References
-
- Ueno A, Oh-ishi S. Critical roles for bradykinin and prostanoids in acute inflammatory reactions: a search using experimental animal models. Current Drug Targets: Inflammation and Allergy. 2002;1(4):363–376. - PubMed
-
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43(2):109–142. - PubMed
-
- Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis and Rheumatism. 1998;41(7):1141–1151. - PubMed
-
- Yaren H, Mollaoglu H, Kurt B, et al. Lung toxicity of nitrogen mustard may be mediated by nitric oxide and peroxynitrite in rats. Research in Veterinary Science. 2007;83(1):116–122. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

