Detection of recurrence by 18F-FDG PET in patients with endometrial cancer showing no evidence of disease
- PMID: 20592894
- PMCID: PMC2890879
- DOI: 10.3346/jkms.2010.25.7.1029
Detection of recurrence by 18F-FDG PET in patients with endometrial cancer showing no evidence of disease
Abstract
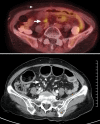
This study assessed the feasibility of F-18-fluorodeoxyglucose positron-emission tomography ((18)F-FDG PET) in the post-therapy surveillance for patients with endometrial cancer showing no evidence of disease (NED). From April 1997 to June 2007, 127 patients with endometrial cancer showing NED were performed (18)F-FDG PET scan. The feasibility of (18)F-FDG PET for the early detection of recurrence in patients with endometrial cancer was evaluated retrospectively. Of the 127 patients, 32 patients showed positive lesions on (18)F-FDG PET scan. Nineteen (19/127 cases, 15%) of them were confirmed to have a recurrence clinically or histologically. The sensitivity, specificity and positive and negative predictive value of (18)F-FDG PET for detecting recurrences in patients with endometrial cancer were 100%, 88%, 59% and 100%, respectively. In conclusion, (18)F-FDG PET may be a useful method for the post-therapy surveillance in patients with endometrial cancer.
Keywords: Endometrial Neoplasms; Positron-Emission Tomography; Recurrence.
Figures
Similar articles
-
18F-FDG PET in the management of endometrial cancer.Eur J Nucl Med Mol Imaging. 2006 Jan;33(1):36-44. doi: 10.1007/s00259-005-1876-y. Epub 2005 Sep 16. Eur J Nucl Med Mol Imaging. 2006. PMID: 16167154
-
[Value of (18)F-FDG imaging and serum tumor markers in the diagnosis of recurrent endometrial carcinoma].Zhonghua Zhong Liu Za Zhi. 2010 Apr;32(4):300-3. Zhonghua Zhong Liu Za Zhi. 2010. PMID: 20510085 Chinese.
-
Clinical and prognostic value of 18F-FDG PET/CT in recurrent endometrial carcinoma.Rev Esp Med Nucl Imagen Mol (Engl Ed). 2019 Mar-Apr;38(2):87-93. doi: 10.1016/j.remn.2018.09.005. Epub 2018 Dec 17. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2019. PMID: 30573388 English, Spanish.
-
[FDG-PET and endometrial cancer].Bull Cancer. 2012 Jan;99(1):21-8. doi: 10.1684/bdc.2011.1514. Bull Cancer. 2012. PMID: 22182739 Review. French.
-
Diagnostic accuracy of preoperative 18F-FDG PET or PET/CT in detecting pelvic and para-aortic lymph node metastasis in patients with endometrial cancer: a systematic review and meta-analysis.Arch Gynecol Obstet. 2019 Sep;300(3):519-529. doi: 10.1007/s00404-019-05207-8. Epub 2019 Jun 4. Arch Gynecol Obstet. 2019. PMID: 31165242
Cited by
-
Development and validation of a circulating tumor DNA-based optimization-prediction model for short-term postoperative recurrence of endometrial cancer.World J Clin Cases. 2024 Jun 26;12(18):3385-3394. doi: 10.12998/wjcc.v12.i18.3385. World J Clin Cases. 2024. PMID: 38983398 Free PMC article.
-
Comparison of 18F-FDG PET/MRI and MRI alone for whole-body staging and potential impact on therapeutic management of women with suspected recurrent pelvic cancer: a follow-up study.Eur J Nucl Med Mol Imaging. 2018 Apr;45(4):622-629. doi: 10.1007/s00259-017-3881-3. Epub 2017 Nov 21. Eur J Nucl Med Mol Imaging. 2018. PMID: 29164299
-
Diagnostic role of 18F-FDG PET/MRI in patients with gynecological malignancies of the pelvis: A systematic review and meta-analysis.PLoS One. 2017 May 8;12(5):e0175401. doi: 10.1371/journal.pone.0175401. eCollection 2017. PLoS One. 2017. PMID: 28481958 Free PMC article.
References
-
- Irvin WP, Rice LW, Berkowitz RS. Advances in the management of endometrial adenocarcinoma. A review. J Reprod Med. 2002;47:173–189. - PubMed
-
- John RL. Uterine Cancer. In: Jonathan SB, editor. Berek & Novak's Gynecology. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 1377.
-
- Sears JD, Greven KM, Hoen HM, Randall ME. Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer. 1994;74:1303–1308. - PubMed
-
- Israel O, Kuten A. Early detection of cancer recurrence: 18F-FDG PET/CT can make a difference in diagnosis and patient care. J Nucl Med. 2007;48(Suppl 1):28S–35S. - PubMed
-
- Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101:520–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

