Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan
- PMID: 20592950
- PMCID: PMC2893712
- DOI: 10.3389/fncel.2010.00016
Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan
Abstract
Functional maturation of visual cortex is linked with dynamic changes in synaptic expression of GABAergic mechanisms. These include setting the excitation-inhibition balance required for experience-dependent plasticity, as well as, intracortical inhibition underlying development and aging of receptive field properties. Animal studies have shown that there is developmental regulation of GABAergic mechanisms in visual cortex. In this study, we show for the first time how these mechanisms develop in the human visual cortex across the lifespan. We used Western blot analysis of postmortem tissue from human primary visual cortex (n = 30, range: 20 days to 80 years) to quantify expression of eight pre- and post-synaptic GABAergic markers. We quantified the inhibitory modulating cannabinoid receptor (CB1), GABA vesicular transporter (VGAT), GABA synthesizing enzymes (GAD65/GAD67), GABA(A) receptor anchoring protein (Gephyrin), and GABA(A) receptor subunits (GABA(A)alpha1, GABA(A)alpha2, GABA(A)alpha3). We found a complex pattern of different developmental trajectories, many of which were prolonged and continued well into the teen, young adult, and even older adult years. These included a monotonic increase or decrease (GABA(A)alpha1, GABA(A)alpha2), a biphasic increase then decrease (GAD65, Gephyrin), or multiple increases and decreases (VGAT, CB1) across the lifespan. Comparing the balances between the pre- and post-synaptic markers we found three main transition stages (early childhood, early teen years, aging) when there were rapid switches in the composition of the GABAergic signaling system, indicating that functioning of the GABAergic system must change as the visual cortex develops and ages. Furthermore, these results provide key information for translating therapies developed in animal models into effective treatments for amblyopia in humans.
Keywords: GABA; aging; development; human; inhibition; plasticity; visual cortex.
Figures
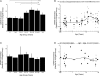
 all runs). Example blots for each case are presented above the scattergrams (B,D). The arrow indicates the band that was quantified. A weighted average curve was fit to each scatter plots using the locally weighted least squares method at 50% (dotted line). GAD65 showed a significant change across the lifespan (Kruskal–Wallis, p < 0.0001) with progressive increase in expression levels into the teenage and adult years then a loss with aging (A,B). There was no change in expression levels of GAD67 across the lifespan (C,D). The lines above the histograms identify the groups that were significantly different (Tukey's post hoc HSD, *p < 0.05, **p < 0.02, ***p < 0.001).
all runs). Example blots for each case are presented above the scattergrams (B,D). The arrow indicates the band that was quantified. A weighted average curve was fit to each scatter plots using the locally weighted least squares method at 50% (dotted line). GAD65 showed a significant change across the lifespan (Kruskal–Wallis, p < 0.0001) with progressive increase in expression levels into the teenage and adult years then a loss with aging (A,B). There was no change in expression levels of GAD67 across the lifespan (C,D). The lines above the histograms identify the groups that were significantly different (Tukey's post hoc HSD, *p < 0.05, **p < 0.02, ***p < 0.001).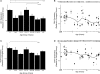
 all runs; dotted line is the weighted average).
all runs; dotted line is the weighted average).
 all runs; dotted line is the weighted average).
all runs; dotted line is the weighted average).
 all runs).
all runs).
 all runs; solid lines are exponential decay functions).
all runs; solid lines are exponential decay functions).
 all runs; dotted line is the weighted average).
all runs; dotted line is the weighted average).Similar articles
-
Development of Glutamatergic Proteins in Human Visual Cortex across the Lifespan.J Neurosci. 2017 Jun 21;37(25):6031-6042. doi: 10.1523/JNEUROSCI.2304-16.2017. Epub 2017 May 29. J Neurosci. 2017. PMID: 28554889 Free PMC article.
-
Vesicular GABA Transporter Is Necessary for Transplant-Induced Critical Period Plasticity in Mouse Visual Cortex.J Neurosci. 2019 Apr 3;39(14):2635-2648. doi: 10.1523/JNEUROSCI.1253-18.2019. Epub 2019 Jan 31. J Neurosci. 2019. PMID: 30705101 Free PMC article.
-
Experience-dependent changes in excitatory and inhibitory receptor subunit expression in visual cortex.Front Synaptic Neurosci. 2010 Sep 28;2:138. doi: 10.3389/fnsyn.2010.00138. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423524 Free PMC article.
-
[Schizophrenia and cortical GABA neurotransmission].Seishin Shinkeigaku Zasshi. 2010;112(5):439-52. Seishin Shinkeigaku Zasshi. 2010. PMID: 20560363 Review. Japanese.
-
GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon.Psychopharmacology (Berl). 2005 Jul;180(2):191-205. doi: 10.1007/s00213-005-2212-8. Epub 2005 Apr 28. Psychopharmacology (Berl). 2005. PMID: 15864560 Review.
Cited by
-
Sensitive periods in fear learning and memory.Stress. 2014 Jan;17(1):13-21. doi: 10.3109/10253890.2013.796355. Epub 2013 May 28. Stress. 2014. PMID: 23611461 Free PMC article. Review.
-
Frequency of gamma oscillations in humans is modulated by velocity of visual motion.J Neurophysiol. 2015 Jul;114(1):244-55. doi: 10.1152/jn.00232.2015. Epub 2015 Apr 29. J Neurophysiol. 2015. PMID: 25925324 Free PMC article.
-
GABA System Modifications During Periods of Hormonal Flux Across the Female Lifespan.Front Behav Neurosci. 2022 Jun 16;16:802530. doi: 10.3389/fnbeh.2022.802530. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35783228 Free PMC article. Review.
-
Trajectory of the main GABAergic interneuron populations from early development to old age in the rat primary auditory cortex.Front Neuroanat. 2014 Jun 2;8:40. doi: 10.3389/fnana.2014.00040. eCollection 2014. Front Neuroanat. 2014. PMID: 24917792 Free PMC article.
-
Development of Glutamatergic Proteins in Human Visual Cortex across the Lifespan.J Neurosci. 2017 Jun 21;37(25):6031-6042. doi: 10.1523/JNEUROSCI.2304-16.2017. Epub 2017 May 29. J Neurosci. 2017. PMID: 28554889 Free PMC article.
References
LinkOut - more resources
Full Text Sources

