Postnatal PPARdelta activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice
- PMID: 20593012
- PMCID: PMC2892469
- DOI: 10.1371/journal.pone.0011307
Postnatal PPARdelta activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice
Abstract
Background: Interventions for T2DM have in part aimed to mimic exercise. Here, we have compared the independent and combined effects of a PPARdelta agonist and endurance training mimetic (GW501516) and a myostatin antibody and resistance training mimetic (PF-879) on metabolic and performance outcomes in obese insulin resistant mice.
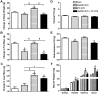
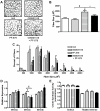
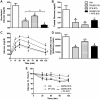
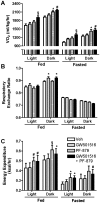
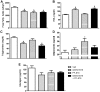
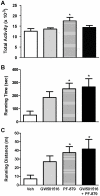
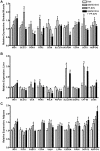
Methodology/principal findings: Male ob/ob mice were treated for 6 weeks with vehicle, GW501516, PF-879, or GW501516 in combination with PF-879. The effects of the interventions on body composition, glucose homeostasis, glucose tolerance, energy expenditure, exercise capacity and metabolic gene expression were compared at the end of study. GW501516 attenuated body weight and fat mass accumulation and increased the expression of genes of oxidative metabolism. In contrast, PF-879 increased body weight by driving muscle growth and altered the expression of genes involved in insulin signaling and glucose metabolism. Despite their differences, both interventions alone improved glucose homeostasis. Moreover, GW501516 more effectively improved serum lipids, and PF-879 uniquely increased energy expenditure, exercise capacity and adiponectin levels. When combined the robust effects of GW501516 and/or PF-879 on body weight, adiposity, muscle mass, glycemia, serum lipids, energy expenditure and exercise capacity were highly conserved.
Conclusions/significance: The data, for the first time, demonstrate postnatal inhibition of myostatin not only promotes gains in muscle mass similar to resistance training,but improves metabolic homeostasis. In several instances, these effects were either distinct from or complimentary to those of GW501516. The data further suggest that strategies to increase muscle mass, and not necessarily oxidative capacity, may effectively counter insulin resistance and T2DM.
Conflict of interest statement
Figures
Similar articles
-
PPARδ agonists have opposing effects on insulin resistance in high fat-fed rats and mice due to different metabolic responses in muscle.Br J Pharmacol. 2011 Jun;163(3):556-66. doi: 10.1111/j.1476-5381.2011.01240.x. Br J Pharmacol. 2011. PMID: 21265823 Free PMC article.
-
Myostatin and adipokines: The role of the metabolically unhealthy obese phenotype in muscle function and aerobic capacity in young adults.Cytokine. 2018 Jul;107:118-124. doi: 10.1016/j.cyto.2017.12.008. Epub 2017 Dec 13. Cytokine. 2018. PMID: 29246653
-
Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity.PLoS One. 2009;4(3):e4937. doi: 10.1371/journal.pone.0004937. Epub 2009 Mar 19. PLoS One. 2009. PMID: 19295913 Free PMC article.
-
Enhanced skeletal muscle for effective glucose homeostasis.Prog Mol Biol Transl Sci. 2014;121:133-63. doi: 10.1016/B978-0-12-800101-1.00005-3. Prog Mol Biol Transl Sci. 2014. PMID: 24373237 Review.
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
Cited by
-
Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit.Nat Rev Endocrinol. 2020 Sep;16(9):495-505. doi: 10.1038/s41574-020-0377-1. Epub 2020 Jul 6. Nat Rev Endocrinol. 2020. PMID: 32632275 Review.
-
Myostain is involved in ginsenoside Rb1-mediated anti-obesity.Pharm Biol. 2022 Dec;60(1):1106-1115. doi: 10.1080/13880209.2022.2074056. Pharm Biol. 2022. PMID: 35639355 Free PMC article.
-
Building muscle, browning fat and preventing obesity by inhibiting myostatin.Diabetologia. 2012 Jan;55(1):13-7. doi: 10.1007/s00125-011-2361-8. Epub 2011 Nov 6. Diabetologia. 2012. PMID: 22057197
-
The correlation of resistance exercise-induced myostatin with insulin resistance and plasma cytokines in healthy young men.J Endocrinol Invest. 2016 Apr;39(4):383-8. doi: 10.1007/s40618-015-0373-9. Epub 2015 Aug 18. J Endocrinol Invest. 2016. PMID: 26280319 Clinical Trial.
-
Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice.Diabetologia. 2012 Jan;55(1):183-93. doi: 10.1007/s00125-011-2304-4. Epub 2011 Sep 17. Diabetologia. 2012. PMID: 21927895
References
-
- Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol. 2008;59(Suppl 7):5–18. - PubMed
-
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. - PubMed
-
- Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, et al. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle -insight from analysis of a transgenic mouse model. Diabetes Res Clin Pract. 2007;77(Suppl 1):S92–98. - PubMed
-
- Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, et al. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. - PubMed
-
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta ), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–1648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

