Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice
- PMID: 20593020
- PMCID: PMC2892485
- DOI: 10.1371/journal.pone.0011256
Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice
Abstract
Background: Liver fibrosis is characterized by excessive synthesis of extracellular matrix proteins, which prevails over their enzymatic degradation, primarily by matrix metalloproteinases (MMPs). The effect of pharmacological MMP inhibition on fibrogenesis, however, is largely unexplored. Inflammation is considered a prerequisite and important co-contributor to fibrosis and is, in part, mediated by tumor necrosis factor (TNF)-alpha-converting enzyme (TACE). We hypothesized that treatment with a broad-spectrum MMP and TACE-inhibitor (Marimastat) would ameliorate injury and inflammation, leading to decreased fibrogenesis during repeated hepatotoxin-induced liver injury.
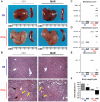
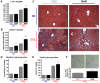
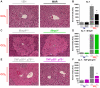
Methodology/principal findings: Liver fibrosis was induced in mice by repeated carbon tetrachloride (CCl4) administration, during which the mice received either Marimastat or vehicle twice daily. A single dose of CCl4 was administered to investigate acute liver injury in mice pretreated with Marimastat, mice deficient in Mmp9, or mice deficient in both TNF-alpha receptors. Liver injury was quantified by alanine aminotransferase (ALT) levels and confirmed by histology. Hepatic collagen was determined as hydroxyproline, and expression of fibrogenesis and fibrolysis-related transcripts was determined by quantitative reverse-transcription polymerase chain reaction. Marimastat-treated animals demonstrated significantly attenuated liver injury and inflammation but a 25% increase in collagen deposition. Transcripts related to fibrogenesis were significantly less upregulated compared to vehicle-treated animals, while MMP expression and activity analysis revealed efficient pharmacologic MMP-inhibition and decreased fibrolysis following Marimastat treatment. Marimastat pre-treatment significantly attenuated liver injury following acute CCl4-administration, whereas Mmp9 deficient animals demonstrated no protection. Mice deficient in both TNF-alpha receptors exhibited an 80% reduction of serum ALT, confirming the hepatoprotective effects of Marimastat via the TNF-signaling pathway.
Conclusions/significance: Inhibition of MMP and TACE activity with Marimastat during chronic CCl4 administration counterbalanced any beneficial anti-inflammatory effect, resulting in a positive balance of collagen deposition. Since effective inhibition of MMPs accelerates fibrosis progression, MMP inhibitors should be used with caution in patients with chronic liver diseases.
Conflict of interest statement
Figures
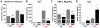




Similar articles
-
Tumor necrosis factor α-converting enzyme inhibition reverses hepatic steatosis and improves insulin sensitivity markers and surgical outcome in mice.PLoS One. 2011;6(9):e25587. doi: 10.1371/journal.pone.0025587. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980496 Free PMC article.
-
A critical role for matrix metalloproteinases in liver regeneration.J Surg Res. 2008 Apr;145(2):192-8. doi: 10.1016/j.jss.2007.04.002. Epub 2008 Jan 28. J Surg Res. 2008. PMID: 18222481 Free PMC article.
-
Glycoprotein Nonmetastatic Melanoma B (Gpnmb)-Positive Macrophages Contribute to the Balance between Fibrosis and Fibrolysis during the Repair of Acute Liver Injury in Mice.PLoS One. 2015 Nov 23;10(11):e0143413. doi: 10.1371/journal.pone.0143413. eCollection 2015. PLoS One. 2015. PMID: 26599547 Free PMC article.
-
Hepatoprotective effect of total flavonoids of Mallotus apelta (Lour.) Muell.Arg. leaf against carbon tetrachloride-induced liver fibrosis in rats via modulation of TGF-β1/Smad and NF-κB signaling pathways.J Ethnopharmacol. 2020 May 23;254:112714. doi: 10.1016/j.jep.2020.112714. Epub 2020 Feb 24. J Ethnopharmacol. 2020. PMID: 32105750
-
Matrix metalloproteinase inhibitors.Invest New Drugs. 1997;15(1):61-75. doi: 10.1023/a:1005722729132. Invest New Drugs. 1997. PMID: 9195290 Review.
Cited by
-
Regulation and involvement of matrix metalloproteinases in vascular diseases.Front Biosci (Landmark Ed). 2016 Jan 1;21(1):89-118. doi: 10.2741/4378. Front Biosci (Landmark Ed). 2016. PMID: 26709763 Free PMC article. Review.
-
Metalloproteinases (MMPs) in hypertensive disorders: role, function, pharmacology, and potential strategies to mitigate pathophysiological changes.Front Pharmacol. 2025 May 26;16:1559288. doi: 10.3389/fphar.2025.1559288. eCollection 2025. Front Pharmacol. 2025. PMID: 40492135 Free PMC article. Review.
-
The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment.Molecules. 2019 Nov 19;24(22):4188. doi: 10.3390/molecules24224188. Molecules. 2019. PMID: 31752262 Free PMC article. Review.
-
Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture.J Infect Dis. 2011 Jun 15;203(12):1753-62. doi: 10.1093/infdis/jir186. J Infect Dis. 2011. PMID: 21606534 Free PMC article.
-
A review of liver fibrosis and cirrhosis regression.J Pathol Transl Med. 2023 Jul;57(4):189-195. doi: 10.4132/jptm.2023.05.24. Epub 2023 Jun 20. J Pathol Transl Med. 2023. PMID: 37461143 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

