Peptide reporters of kinase activity in whole cell lysates
- PMID: 20593469
- PMCID: PMC2914461
- DOI: 10.1002/bip.21401
Peptide reporters of kinase activity in whole cell lysates
Abstract
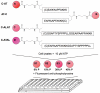
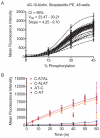
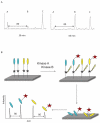
Kinase assays are used to screen for small-molecule inhibitors that may show promise as targeted pharmaceutical therapies. Using cell lysates instead of purified kinases provides a more accurate estimate of inhibitor sensitivity and selectivity in a biological setting. This review summarizes the range of homogeneous (solution-phase) and heterogeneous (solid-supported) formats available for using peptide substrates to monitor kinase activities in cell lysates. With a focus on heterogeneous kinase assays, the peptide substrate Abltide is used as a model to optimize presentation geometries and the modular arrangement of short sequences for kinase recognition. We present results from peptides immobilized on two- and three-dimensional surfaces such as hydrogels on 96-well plates and glass slides, and fluorescent Luminex beads. We discuss methods to increase assay sensitivity using chemifluorescent ELISAs, antibody-based recognition, and label-free mass spectrometry. Monitoring the activity of specific kinases in cell lysates presents challenges that can be overcome by manipulating peptide substrates to optimize assay conditions. In particular, signal-to-background ratios were improved by (1) adding long branched hydrophilic linkers between the substrate and the surface, (2) changing the orientation of peptides relative to the surface, and (3) including peptide ligands in cis or in trans to recruit kinases to the surface. By improving the accessibility of immobilized peptide substrates to kinases in solution, the apparent rate of phosphorylation increased and assays were more sensitive to changes in endogenous kinase activities. These strategies can be generalized to improve the reactivity of most peptide substrates used in heterogeneous kinase assays with cell lysates.
Copyright (c) 2010 Wiley Periodicals, Inc.
Figures




References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. - PubMed
-
- Gray N, Detivaud L, Doerig C, Meijer L. ATP-site directed inhibitors of cyclin-dependent kinases. Curr Med Chem. 1999;6(9):859–875. - PubMed
-
- Sawyers C. Targeted cancer therapy. Nature. 2004;432(7015):294–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

