Total chemical synthesis of human T-cell leukemia virus type 1 protease via native chemical ligation
- PMID: 20593478
- PMCID: PMC2917242
- DOI: 10.1002/bip.21375
Total chemical synthesis of human T-cell leukemia virus type 1 protease via native chemical ligation
Abstract
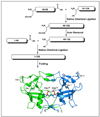
Human T-cell leukemia virus 1 (HTLV-1) protease, a member of the aspartic acid protease family, plays critical roles in the pathogenesis of the virus and is an attractive viral target for therapeutic intervention. HTLV-1 protease consists of 125 amino acid residues and functions as a homodimer stabilized in part by a four-stranded beta-sheet comprising the N- and C-termini. Compared with many other viral proteases such as HIV-1 protease, HTLV-1 protease is elongated by an extra 10 amino acid residue "tail" at the C-terminus. The structural and functional role of the extra C-terminal residues in the catalysis of HTLV-1 protease has been a subject of debate for years. Using the native chemical ligation technique pioneered by Kent and coworkers, we chemically synthesized a full-length HTLV protease and a C-terminally truncated form encompassing residues 1-116. Enzyme kinetic analysis using three different peptide substrates indicated that truncation of the C-terminal tail lowered the turnover number of the viral enzyme by a factor of 2 and its catalytic efficiency by roughly 10-fold. Our findings differ from the two extreme views that the C-terminal tail of HTLV-1 protease is either fully dispensable or totally required for enzyme dimerization and/or catalysis.
Copyright (c) 2010 Wiley Periodicals, Inc.
Figures




Similar articles
-
C-terminal residues of mature human T-lymphotropic virus type 1 protease are critical for dimerization and catalytic activity.Biochem J. 2008 Dec 15;416(3):357-64. doi: 10.1042/BJ20071132. Biochem J. 2008. PMID: 18636969 Free PMC article.
-
Characterizing the protonation states of the catalytic residues in apo and substrate-bound human T-cell leukemia virus type 1 protease.Comput Biol Chem. 2015 Jun;56:61-70. doi: 10.1016/j.compbiolchem.2015.04.002. Epub 2015 Apr 4. Comput Biol Chem. 2015. PMID: 25889320
-
Human T-cell leukemia virus type 1 protease protein expressed in Escherichia coli possesses aspartic proteinase activity.Arch Virol. 1993;128(3-4):195-210. doi: 10.1007/BF01309434. Arch Virol. 1993. PMID: 8435041
-
The protease of human T-cell leukemia virus type-1 is a potential therapeutic target.Curr Pharm Des. 2007;13(12):1285-94. doi: 10.2174/138161207780618849. Curr Pharm Des. 2007. PMID: 17504236 Review.
-
Design of inhibitors against HIV, HTLV-I, and Plasmodium falciparum aspartic proteases.Biol Chem. 2004 Nov;385(11):1035-9. doi: 10.1515/BC.2004.134. Biol Chem. 2004. PMID: 15576323 Review.
Cited by
-
Total chemical synthesis, refolding, and crystallographic structure of fully active immunophilin calstabin 2 (FKBP12.6).Protein Sci. 2016 Dec;25(12):2225-2242. doi: 10.1002/pro.3051. Epub 2016 Oct 13. Protein Sci. 2016. PMID: 27670942 Free PMC article.
-
Total chemical synthesis of human interferon alpha-2b via native chemical ligation.J Pept Sci. 2015 Jul;21(7):554-60. doi: 10.1002/psc.2760. Epub 2015 Mar 24. J Pept Sci. 2015. PMID: 25810135 Free PMC article.
-
Interrogation of MDM2 phosphorylation in p53 activation using native chemical ligation: the functional role of Ser17 phosphorylation in MDM2 reexamined.J Am Chem Soc. 2012 Apr 18;134(15):6855-64. doi: 10.1021/ja301255n. Epub 2012 Apr 4. J Am Chem Soc. 2012. PMID: 22444248 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

