Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial
- PMID: 20593498
- PMCID: PMC2896750
- DOI: 10.3748/wjg.v16.i25.3133
Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial
Abstract
Aim: To investigate efficacy and safety of cetuximab combined with two chemotherapy regimens in patients with unresectable metastatic colorectal cancer (mCRC).
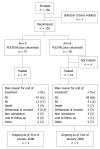
Methods: Randomized patients received cetuximab with 5-fluorouracil (5-FU), folinic acid (FA) and oxaliplatin (FOLFOX) 6 (arm A, n = 74) or 5-FU, FA and irinotecan (FOLFIRI) (arm B, n = 77). KRAS mutation status was determined retrospectively in a subset of tumors (n = 117).
Results: No significant difference was found between treatment arms A and B in the progression-free survival (PFS) rate at 9 mo, 45% vs 34%; median PFS, 8.6 mo vs 8.3 mo [hazard ratio (HR) = 1.06]; overall response rate (ORR) 43% vs 45% [odds ratio (OR) = 0.93] and median overall survival (OS), 17.4 mo vs 18.9 mo (HR = 0.98). Patients with KRAS wild-type tumors demonstrated improved PFS (HR = 0.55, P = 0.0051), OS, (HR = 0.62, P = 0.0296) and ORR (53% vs 36%) and in arm A, improved PFS (HR = 0.49, P = 0.0196), OS (HR = 0.48, P = 0.0201) and ORR (56% vs 30%), compared with patients with KRAS mutated tumors. In arm B no significant differences were found in efficacy by KRAS mutation status. Treatment in arms A and B was generally well tolerated.
Conclusion: This study confirms that combinations of cetuximab with FOLFOX6 or FOLFIRI are effective and significantly improve clinical outcome in KRAS wild-type compared with KRAS mutated mCRC.
Figures


Similar articles
-
Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer.J Clin Oncol. 2010 Nov 1;28(31):4706-13. doi: 10.1200/JCO.2009.27.6055. Epub 2010 Oct 4. J Clin Oncol. 2010. PMID: 20921462 Clinical Trial.
-
Activating KRAS mutations and overexpression of epidermal growth factor receptor as independent predictors in metastatic colorectal cancer patients treated with cetuximab.Ann Surg. 2010 Feb;251(2):254-60. doi: 10.1097/SLA.0b013e3181bc9d96. Ann Surg. 2010. PMID: 20010090
-
FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306.Ann Oncol. 2012 Jul;23(7):1693-9. doi: 10.1093/annonc/mdr571. Epub 2012 Jan 4. Ann Oncol. 2012. PMID: 22219013 Clinical Trial.
-
Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome.Clin Transl Oncol. 2010 Aug;12(8):533-42. doi: 10.1007/s12094-010-0551-3. Clin Transl Oncol. 2010. PMID: 20709651 Review.
-
Chemotherapy of metastatic colorectal cancer.Prescrire Int. 2010 Oct;19(109):219-24. Prescrire Int. 2010. PMID: 21180382 Review.
Cited by
-
Efficacy and clinical monitoring strategies for immune checkpoint inhibitors and targeted cytokine immunotherapy for locally advanced and metastatic colorectal cancer.Cytokine Growth Factor Rev. 2019 Oct;49:1-9. doi: 10.1016/j.cytogfr.2019.10.002. Epub 2019 Oct 24. Cytokine Growth Factor Rev. 2019. PMID: 31679887 Free PMC article. Review.
-
Cost-effectiveness Analysis of Cetuximab in Treatment of Metastatic Colorectal Cancer in Iranian Pharmaceutical Market.Int J Prev Med. 2015 Jul 16;6:63. doi: 10.4103/2008-7802.161068. eCollection 2015. Int J Prev Med. 2015. PMID: 26288707 Free PMC article.
-
The role of cetuximab as first-line treatment of colorectal liver metastases.HPB (Oxford). 2013 Jan;15(1):11-7. doi: 10.1111/j.1477-2574.2012.00591.x. Epub 2012 Oct 17. HPB (Oxford). 2013. PMID: 23216774 Free PMC article. Review.
-
Current therapy of advanced colorectal cancer according to RAS/RAF mutational status.Cancer Metastasis Rev. 2020 Dec;39(4):1143-1157. doi: 10.1007/s10555-020-09913-7. Cancer Metastasis Rev. 2020. PMID: 32648137 Review.
-
Economic Evaluation of Monoclonal Antibodies in Metastatic Colorectal Cancer: A Systematic Review.Mol Diagn Ther. 2021 Nov;25(6):715-734. doi: 10.1007/s40291-021-00560-4. Epub 2021 Nov 24. Mol Diagn Ther. 2021. PMID: 34816395
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O'Connell M, Sargent P, Piedbois P. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766–3775. - PubMed
-
- Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. - PubMed
-
- Borner MM, Castiglione M, Bacchi M, Weber W, Herrmann R, Fey MF, Pagani O, Leyvraz S, Morant R, Pestalozzi B, et al. The impact of adding low-dose leucovorin to monthly 5-fluorouracil in advanced colorectal carcinoma: results of a phase III trial. Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 1998;9:535–541. - PubMed
-
- Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–1418. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

