Synthesis and conformational analysis of bicyclic extended dipeptide surrogates
- PMID: 20593836
- PMCID: PMC2914495
- DOI: 10.1021/jo1008433
Synthesis and conformational analysis of bicyclic extended dipeptide surrogates
Abstract
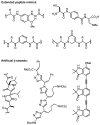
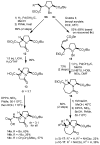
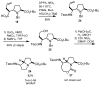
Regio- and diastereoselective reactions of a homoproline enolate enable the synthesis of novel extended dipeptide surrogates. Bicyclic carbamate 9 and fused beta-lactam scaffold 11 were prepared from L-pyroglutamic acid via substrate-controlled electrophilic azidation. Synthesis of orthogonally protected hexahydropyrrolizine, hexahydropyrrolizinone, and hexahydropyrroloazepinone dipeptide surrogates relied on allylation of proline derivative 5, followed by Curtius rearrangement to introduce the N-terminal carbamate group. A total of six azabicycloalkane derivatives were evaluated for conformational mimicry of extended dipeptides by a combination of X-ray diffraction and molecular modeling. Analysis of putative backbone dihedral angles and N- to C-terminal dipeptide distances indicate that compounds (alpha'S)-14b and 21 approximate the conformation of dipeptides found in beta-sheets, while tripeptide mimic 28 is also highly extended in the solid state. Structural data suggest that ring size and relative stereochemistry have a profound effect on the ability of these scaffolds to act as beta-strand mimetics and should inform the design of related conformational probes.
Figures


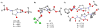




Similar articles
-
Azabicycloalkenes as synthetic intermediates: application to the preparation of diazabicycloalkane scaffolds.Org Lett. 2006 Apr 13;8(8):1681-4. doi: 10.1021/ol060287q. Org Lett. 2006. PMID: 16597140
-
Phe-Ala-based diazaspirocyclic lactam as nucleator of type II' β-turn.J Org Chem. 2011 Feb 4;76(3):833-9. doi: 10.1021/jo1019927. Epub 2011 Jan 11. J Org Chem. 2011. PMID: 21222472
-
Constrained Dipeptide Surrogates: 5- and 7-Hydroxy Indolizidin-2-one Amino Acid Synthesis from Iodolactonization of Dehydro-2,8-diamino Azelates.Molecules. 2021 Dec 23;27(1):67. doi: 10.3390/molecules27010067. Molecules. 2021. PMID: 35011297 Free PMC article.
-
Design, synthesis, and application of azabicyclo[X.Y.0]alkanone amino acids as constrained dipeptide surrogates and peptide mimics.Biopolymers. 2005;80(2-3):98-150. doi: 10.1002/bip.20213. Biopolymers. 2005. PMID: 15795926 Review.
-
Chiral dipeptide mimics possessing a fluoroolefin moiety: a relevant tool for conformational and medicinal studies.Org Biomol Chem. 2007 Apr 21;5(8):1151-7. doi: 10.1039/b701559c. Epub 2007 Mar 19. Org Biomol Chem. 2007. PMID: 17406709 Review.
Cited by
-
Substituted imidazo[1,2-a]pyridines as β-strand peptidomimetics.Org Lett. 2012 Dec 21;14(24):6162-5. doi: 10.1021/ol302850n. Epub 2012 Dec 4. Org Lett. 2012. PMID: 23210770 Free PMC article.
-
Mechanism of the C5 stereoinversion reaction in the biosynthesis of carbapenem antibiotics.Science. 2014 Mar 7;343(6175):1140-4. doi: 10.1126/science.1248000. Science. 2014. PMID: 24604200 Free PMC article.
References
-
- Kemp DS, Bowen BR, Muendel CC. J Org Chem. 1990;55:4650–4657.
- Khakshoor O, Nowick JS. Curr Opin Chem Biol. 2008;12:722–729. - PMC - PubMed
- Levin S, Nowick JS. J Am Chem Soc. 2007;129:13043–13048. - PMC - PubMed
- Nowick JS. Acc Chem Res. 1999;32:287–296.
- Nowick JS, Smith EM, Noronha G. J Org Chem. 1995;60:7386–7387.
- Nowick JS, Cary JM, Tsai JH. J Am Chem Soc. 2001;123:5176–5180. - PubMed
- Phillips ST, Rezac M, Abel U, Kossenjans M, Bartlett PA. J Am Chem Soc. 2002;124:58–66. - PubMed
- Zeng H, Yang X, Flowers RA, Gong B. J Am Chem Soc. 2002;124:2903–2910. - PubMed
-
- Smith CK, Regan L. Acc Chem Res. 1997;30:153–161.
- Fairlie DP, Tyndall JDA, Reid RC, Wong AK, Abbenante G, Scanlon MJ, March DR, Bergman DA, Chai CLL, Burkett BA. J Med Chem. 2000;43:1271–1281. - PubMed
- Tyndall JDA, Fairlie DP. J Mol Recog. 1999;12:363–370. - PubMed
- Wolfram B, Robert H. Eur J Biochem. 1992;204:433–451. - PubMed
- Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. Nature. 1993;364:33–39. - PubMed
- Sawyer TK, Bohacek RS, Dalgarno DC, Eyermann CJ, Kawahata N, Metcalf CA, Shakespeare WC, Sundaramoorthi R, Wang Y, Yang MG. Mini-Reviews in Medicinal Chemistry. 2002;2:475–488. - PubMed
-
- Strickland CL, Windsor WT, Syto R, Wang L, Bond R, Wu Z, Schwartz J, Le HV, Beese LS, Weber PC. Biochemistry. 1998;37:16601–16611. - PubMed
- Long SB, Casey PJ, Beese LS. Structure. 2000;8:209–222. - PubMed
- Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Nat Struct Mol Biol. 2002;9:940–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

