Jeffamine derivatized TentaGel beads and poly(dimethylsiloxane) microbead cassettes for ultrahigh-throughput in situ releasable solution-phase cell-based screening of one-bead-one-compound combinatorial small molecule libraries
- PMID: 20593859
- PMCID: PMC3045263
- DOI: 10.1021/cc100083f
Jeffamine derivatized TentaGel beads and poly(dimethylsiloxane) microbead cassettes for ultrahigh-throughput in situ releasable solution-phase cell-based screening of one-bead-one-compound combinatorial small molecule libraries
Abstract
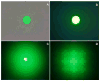
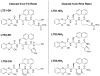
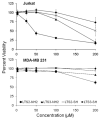
A method to efficiently immobilize and partition large quantities of microbeads in an array format in microfabricated poly(dimethylsiloxane) (PDMS) cassette for ultrahigh-throughput in situ releasable solution-phase cell-based screening of one-bead-one-compound (OBOC) combinatorial libraries is described. Commercially available Jeffamine triamine T-403 (∼440 Da) was derivatized such that two of its amino groups were protected by Fmoc and the remaining amino group capped with succinic anhydride to generate a carboxyl group. This resulting trifunctional hydrophilic polymer was then sequentially coupled two times to the outer layer of topologically segregated bilayer TentaGel (TG) beads with solid phase peptide synthesis chemistry resulting in beads with increased loading capacity, hydrophilicity, and porosity at the outer layer. We have found that such bead configuration can facilitate ultrahigh-throughput in situ releasable solution-phase screening of OBOC libraries. An encoded releasable OBOC small molecule library was constructed on Jeffamine derivatized TG beads with library compounds tethered to the outer layer via a disulfide linker and coding tags in the interior of the beads. Compound-beads could be efficiently loaded (5-10 min) into a 5 cm diameter Petri dish containing a 10,000-well PDMS microbead cassette, such that over 90% of the microwells were each filled with only one compound-bead. Jurkat T-lymphoid cancer cells suspended in Matrigel were then layered over the microbead cassette to immobilize the compound-beads. After 24 h of incubation at 37 °C, dithiothreitol was added to trigger the release of library compounds. Forty-eight hours later, MTT reporter assay was used to identify regions of reduced cell viability surrounding each positive bead. From a total of about 20,000 beads screened, 3 positive beads were detected and physically isolated for decoding. A strong consensus motif was identified for these three positive compounds. These compounds were resynthesized and found to be cytotoxic (IC(50) 50-150 μM) against two T-lymphoma cell lines and less so against the MDA-MB 231 breast cancer cell line. This novel ultrahigh-throughput OBOC releasable method can potentially be adapted to many existing 96- or 384-well solution-phase cell-based or biochemical assays.
Figures

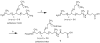

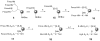

Similar articles
-
Development of hydrogel TentaGel shell-core beads for ultrahigh throughput solution-phase screening of encoded OBOC combinatorial small molecule libraries.J Comb Chem. 2009 Jan-Feb;11(1):91-102. doi: 10.1021/cc800092y. J Comb Chem. 2009. PMID: 19061339 Free PMC article.
-
Encoding method for OBOC small molecule libraries using a biphasic approach for ladder-synthesis of coding tags.J Am Chem Soc. 2004 May 12;126(18):5740-9. doi: 10.1021/ja049322j. J Am Chem Soc. 2004. PMID: 15125667
-
3-nitro-tyrosine as an internal quencher of autofluorescence enhances the compatibility of fluorescence based screening of OBOC combinatorial libraries.Comb Chem High Throughput Screen. 2010 Jun;13(5):422-9. doi: 10.2174/138620710791292994. Comb Chem High Throughput Screen. 2010. PMID: 20236063
-
Design, synthesis, and application of OB2C combinatorial peptide and peptidomimetic libraries.Methods Mol Biol. 2015;1248:3-22. doi: 10.1007/978-1-4939-2020-4_1. Methods Mol Biol. 2015. PMID: 25616322 Free PMC article. Review.
-
Application of combinatorial library methods in cancer research and drug discovery.Anticancer Drug Des. 1997 Apr;12(3):145-67. Anticancer Drug Des. 1997. PMID: 9154108 Review.
Cited by
-
Microfluidic impact printer with interchangeable cartridges for versatile non-contact multiplexed micropatterning.Lab Chip. 2013 May 21;13(10):1902-10. doi: 10.1039/c3lc41372a. Epub 2013 Mar 25. Lab Chip. 2013. PMID: 23525299 Free PMC article.
-
Acid-Promoted Direct C-H Carbamoylation at the C-3 Position of Quinoxalin-2(1H)-ones with Isocyanide in Water.ACS Omega. 2022 Dec 16;8(1):1577-1587. doi: 10.1021/acsomega.2c06946. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643431 Free PMC article.
-
Decorating polymer beads with 1014 inorganic-organic [2]rotaxanes as shown by spin counting.Commun Chem. 2022 Jun 20;5(1):73. doi: 10.1038/s42004-022-00689-1. Commun Chem. 2022. PMID: 36697699 Free PMC article.
-
Development of a large peptoid-DOTA combinatorial library.Biopolymers. 2016 Sep;106(5):673-84. doi: 10.1002/bip.22883. Biopolymers. 2016. PMID: 27257968 Free PMC article.
-
Combinatorial Peptide Microarray Synthesis Based on Microfluidic Impact Printing.ACS Comb Sci. 2019 Jan 14;21(1):6-10. doi: 10.1021/acscombsci.8b00125. Epub 2018 Dec 30. ACS Comb Sci. 2019. PMID: 30521316 Free PMC article.
References
-
- Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354(6348):82–4. - PubMed
-
- Lam KS, Lebl M, Krchnak V, Lake DF, Smith J, Wade S, Ferguson R, Ackerman-Berrier, Wertman K. Application of Selectide Technology in Identifying (i) a Mimotope for a Discontinuous Epitope, and (ii) D-amino Acid Ligands. In: Hodges RS, editor. Peptides: Chemistry, Structure and Biology, (Proc 12th Amer Peptide Symp) 1994. pp. 1003–1004.
- Lam KS, Lebl M, Wade S, Stierandova A, Khattri PS, Collins N, Hruby VJ. Streptavidin-peptide interaction as a model system for molecular recognition. Pept : Chem, Struct Biol, Proc Am Pept Symp 13th. 1994:1005–1006.
-
- Aina OH, Marik J, Liu R, Lau DH, Lam KS. Identification of novel targeting peptides for human ovarian cancer cells using “one-bead one-compound” combinatorial libraries. Mol Cancer Ther. 2005;4:806–813. - PubMed
- Aina OH, Sroka TC, Chen M, Lam KS. Therapeutic cancer targeting peptides. Biopolymers. 2002;66:184–199. - PubMed
- Benito JM, Meldal M. Bicyclic organo-peptides as selective carbohydrate receptors: design, solid-phase synthesis, and on-bead binding capability. QSAR Comb Sci. 2004;23:117–129.
- Lau DH, Guo L, Liu R, Song A, Shao C, Lam KS. Identifying peptide ligands for cell surface receptors using cell-growth-on-bead assay and one-bead one-compound combinatorial library. Biotechnol Lett. 2002;24:497–500.
- McBride JD, Freeman HNM, Leatherbarrow RJ. Selection of human elastase inhibitors from a conformationally constrained combinatorial peptide library. Eur J Biochem. 1999;266:403–412. - PubMed
- McBride JD, Freeman N, Domingo GJ, Leatherbarrow RJ. Selection of chymotrypsin inhibitors from a conformationally-constrained combinatorial peptide library. J Mol Biol. 1996;259:819–827. - PubMed
- Mikawa M, Wang H, Guo L. Novel peptide ligands for integrin a4b1 overexpressed in cancer cells. Mol Cancer Ther. 2004;3:1329–1334. - PubMed
- Wittmann V, Seeberger S. Combinatorial solid-phase synthesis of multivalent cyclic neoglycopeptides. Angew Chem, Int Ed. 2000;39:4348–4352. - PubMed
-
- Liu B, Alluri PG, Yu P, Kodadek T. A Potent Transactivation Domain Mimic with Activity in Living Cells. J Am Chem Soc. 2005;127:8254–8255. - PubMed
- Alluri PG, Reddy MM, Bachhawat-Sikder K, Olivos HJ, Kodadek T. Isolation of protein ligands from large peptoid libraries. J Am Chem Soc. 2003;125:13995–14004. - PubMed
- Reddy MM, Bachhawat-Sikder K, Kodadek T. Transformation of Low-Affinity Lead Compounds into High-Affinity Protein Capture Agents. Chem Biol. 2004;11:1127–1137. - PubMed
-
- Appell KC, Chung TDY, Ohlmeyer MJH, Sigal NH, Baldwin JJ, Chelsky D. Biological screening of a large combinatorial library. J Biomol Screening. 1996;1:27–31.
- Baldwin JJ, Burbaum JJ, Henderson I, Ohlmeyer MHJ. Synthesis of a Small Molecule Combinatorial Library Encoded with Molecular Tags. J Am Chem Soc. 1995;117:5588–5589.
- Lam KS, Liu R, Miyamoto S, Lehman AL, Tuscano JM. Applications of one-bead one-compound combinatorial libraries and chemical microarrays in signal transduction research. Acc Chem Res. 2003;36:370–377. - PubMed
- Lo MMC, Neumann CS, Nagayama S, Perlstein EO, Schreiber SL. A Library of Spirooxindoles Based on a Stereoselective Three-Component Coupling Reaction. J Am Chem Soc. 2004;126:16077–16086. - PubMed
- Stavenger RA, Schreiber SL. Asymmetric catalysis in diversity-oriented organic synthesis: Enantioselective synthesis of 4320 encoded and spatially segregated dihydropyrancarboxamides. Angew Chem, Int Ed. 2001;40:3417–3421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

