Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases
- PMID: 20594269
- PMCID: PMC3745773
- DOI: 10.1111/j.1440-1827.2010.02547.x
Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases
Abstract
Cellular functions within tissues are strictly regulated by the tissue microenvironment which comprises extracellular matrix and extracellular matrix-deposited factors such as growth factors, cytokines and chemokines. These molecules are metabolized by matrix metalloproteinases (MMP), a disintegrin and metalloproteinases (ADAM) and ADAM with thrombospondin motifs (ADAMTS), which are members of the metzincin superfamily. They function in various pathological conditions of both neoplastic and non-neoplastic diseases by digesting different substrates under the control of tissue inhibitors of metalloproteinases (TIMP) and reversion-inducing, cysteine-rich protein with Kazal motifs (RECK). In neoplastic diseases MMP play a central role in cancer cell invasion and metastases, and ADAM are also important to cancer cell proliferation and progression through the metabolism of growth factors and their receptors. Numerous papers have described the involvement of these metalloproteinases in non-neoplastic diseases in nearly every organ. In contrast to the numerous review articles on their roles in cancer cell proliferation and progression, there are very few articles discussing non-neoplastic diseases. This review therefore will focus on the properties of MMP, ADAM and ADAMTS and their implications for non-neoplastic diseases of the cardiovascular system, respiratory system, central nervous system, digestive system, renal system, wound healing and infection, and joints and muscular system.
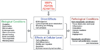
Figures



References
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. - PubMed
-
- Murphy G. The ADAM: Signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. - PubMed
-
- Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22:145–152. - PubMed
-
- Itoh Y, Seiki M. MT1-MMP: A potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

