Identification and characterization of retinoblastoma gene mutations disturbing apoptosis in human breast cancers
- PMID: 20594292
- PMCID: PMC2908580
- DOI: 10.1186/1476-4598-9-173
Identification and characterization of retinoblastoma gene mutations disturbing apoptosis in human breast cancers
Abstract
Background: The tumor suppressor pRb plays a key role regulating cell cycle arrest, and disturbances in the RB1 gene have been reported in different cancer forms. However, the literature reports contradictory findings with respect to a pro--versus anti--apoptotic role of pRb, and the consequence of alterations in RB1 to chemotherapy sensitivity remains unclear. This study is part of a project investigating alterations in pivotal genes as predictive factors to chemotherapy sensitivity in breast cancer.
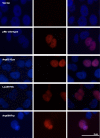
Results: Analyzing 73 locally advanced (stage III) breast cancers, we identified two somatic and one germline single nucleotide changes, each leading to amino acid substitution in the pRb protein (Leu607Ile, Arg698Trp, and Arg621Cys, respectively). This is the first study reporting point mutations affecting RB1 in breast cancer tissue. In addition, MLPA analysis revealed two large multiexon deletions (exons 13 to 27 and exons 21 to 23) with the exons 21-23 deletion occurring in the tumor also harboring the Leu607Ile mutation. Interestingly, Leu607Ile and Arg621Cys point mutations both localize to the spacer region of the pRb protein, a region previously shown to harbor somatic and germline mutations. Multiple sequence alignment across species indicates the spacer to be evolutionary conserved. All three RB1 point mutations encoded nuclear proteins with impaired ability to induce apoptosis compared to wild-type pRb in vitro. Notably, three out of four tumors harboring RB1 mutations displayed primary resistance to treatment with either 5-FU/mitomycin or doxorubicin while only 14 out of 64 tumors without mutations were resistant (p = 0.046).
Conclusions: Although rare, our findings suggest RB1 mutations to be of pathological importance potentially affecting sensitivity to mitomycin/anthracycline treatment in breast cancer.
Figures


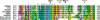



References
-
- Borg A, Zhang QX, Alm P, Olsson H, Sellberg G. The retinoblastoma gene in breast cancer: allele loss is not correlated with loss of gene protein expression. Cancer Res. 1992;52:2991–2994. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

