Regulation of cytokines by small RNAs during skin inflammation
- PMID: 20594301
- PMCID: PMC2905360
- DOI: 10.1186/1423-0127-17-53
Regulation of cytokines by small RNAs during skin inflammation
Abstract
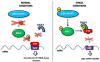
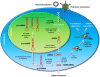
Intercellular signaling by cytokines is a vital feature of the innate immune system. In skin, an inflammatory response is mediated by cytokines and an entwined network of cellular communication between T-cells and epidermal keratinocytes. Dysregulated cytokine production, orchestrated by activated T-cells homing to the skin, is believed to be the main cause of psoriasis, a common inflammatory skin disorder. Cytokines are heavily regulated at the transcriptional level, but emerging evidence suggests that regulatory mechanisms that operate after transcription play a key role in balancing the production of cytokines. Herein, we review the nature of cytokine signaling in psoriasis with particular emphasis on regulation by mRNA destabilizing elements and the potential targeting of cytokine-encoding mRNAs by miRNAs. The proposed linkage between mRNA decay mediated by AU-rich elements and miRNA association is described and discussed as a possible general feature of cytokine regulation in skin. Moreover, we describe the latest attempts to therapeutically target cytokines at the RNA level in psoriasis by exploiting the cellular RNA interference machinery. The applicability of cytokine-encoding mRNAs as future clinical drug targets is evaluated, and advances and obstacles related to topical administration of RNA-based drugs targeting the cytokine circuit in psoriasis are described.
Figures


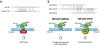
Similar articles
-
Integrated microRNA/mRNA expression profiling of the skin of psoriasis patients.J Dermatol Sci. 2020 Jan;97(1):9-20. doi: 10.1016/j.jdermsci.2019.11.003. Epub 2019 Nov 30. J Dermatol Sci. 2020. PMID: 31843230
-
Innate immunity in the pathogenesis of psoriasis.Arch Dermatol Res. 2011 Dec;303(10):691-705. doi: 10.1007/s00403-011-1169-1. Epub 2011 Aug 24. Arch Dermatol Res. 2011. PMID: 21863252 Review.
-
Cytokines in psoriasis.Cytokine. 2015 Jun;73(2):342-50. doi: 10.1016/j.cyto.2014.12.014. Epub 2015 Jan 10. Cytokine. 2015. PMID: 25585875 Free PMC article. Review.
-
Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes.J Immunol. 2003 Sep 15;171(6):3262-9. doi: 10.4049/jimmunol.171.6.3262. J Immunol. 2003. PMID: 12960356
-
Regulation of pro-inflammatory cytokines TNFα and IL24 by microRNA-203 in primary keratinocytes.Cytokine. 2012 Dec;60(3):741-8. doi: 10.1016/j.cyto.2012.07.031. Epub 2012 Aug 20. Cytokine. 2012. PMID: 22917968
Cited by
-
Ischemia/reperfusion injured intestinal epithelial cells cause cortical neuron death by releasing exosomal microRNAs associated with apoptosis, necroptosis, and pyroptosis.Sci Rep. 2020 Sep 1;10(1):14409. doi: 10.1038/s41598-020-71310-5. Sci Rep. 2020. PMID: 32873851 Free PMC article.
-
Kinetic Cytokine Secretion Profile of LPS-Induced Inflammation in the Human Skin Organ Culture.Pharmaceutics. 2020 Mar 25;12(4):299. doi: 10.3390/pharmaceutics12040299. Pharmaceutics. 2020. PMID: 32218380 Free PMC article.
-
Control by a hair's breadth: the role of microRNAs in the skin.Cell Mol Life Sci. 2013 Apr;70(7):1149-69. doi: 10.1007/s00018-012-1117-z. Epub 2012 Sep 15. Cell Mol Life Sci. 2013. PMID: 22983383 Free PMC article. Review.
-
Utilization of biodegradable polymeric materials as delivery agents in dermatology.Clin Cosmet Investig Dermatol. 2014 Jan 9;7:23-34. doi: 10.2147/CCID.S39559. eCollection 2014. Clin Cosmet Investig Dermatol. 2014. PMID: 24470766 Free PMC article. Review.
-
Orchestrated Cytokines Mediated by Biologics in Psoriasis and Its Mechanisms of Action.Biomedicines. 2022 Feb 20;10(2):498. doi: 10.3390/biomedicines10020498. Biomedicines. 2022. PMID: 35203707 Free PMC article. Review.
References
-
- Sand C, Veien NK. Book Psoriasis (Editor ed.^eds.), vol. LEO TEMABOG. City: Lep Pharma; 2005. Psoriasis.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

