Amphiphilic block co-polyesters bearing pendant cyclic ketal groups as nanocarriers for controlled release of camptothecin
- PMID: 20594408
- PMCID: PMC2974953
- DOI: 10.1163/092050610X504260
Amphiphilic block co-polyesters bearing pendant cyclic ketal groups as nanocarriers for controlled release of camptothecin
Abstract
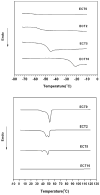
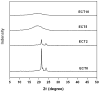
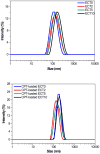
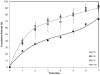
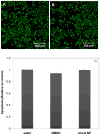
Amphiphilic block co-polymers consisting of hydrophilic poly(ethylene glycol) and hydrophobic polyester bearing pendent cyclic ketals were synthesized by ring-opening co-polymerization of ε-caprolactone (CL) and 1,4,8-trioxaspiro-[4,6]-9-undecanone (TSU) using α-hydroxyl, ω-methoxy, poly(ethylene glycol) as the initiator and stannous octoate as the catalyst. Compositional analyses indicate that TSU was randomly distributed in the hydrophobic blocks. When the TSU content in the co-polymers increased, the polymer crystallinity decreased progressively and the glass transition temperature increased accordingly. The hydrophobic, anticancer drug, camptothecin (CPT), was successfully encapsulated in the block copolymer nanoparticles. The CPT encapsulation efficiency and release kinetics were strongly dependent on the co-polymer composition and crystallinity. CPT release from nanoparticles constructed from co-polymers containing 0, 39 and 100 mol% TSU in the hydrophobic block followed the same trend, with an initial burst of approx. 40% within one day followed by a moderate and slow release lasting up to 7 days. At a TSU content of 14 mol%, CPT was released in a continuous and controlled fashion with a reduced initial burst and a 73% cumulative release by day 7. The in vitro cytoxicity assay showed that the blank nanoparticles were not toxic to the cultured bone metastatic prostate cancer cells (C4-2B). Compared to the free drug, the encapsulated CPT was more effective in inducing apoptotic responses in C4-2B cells. Modulating the physical characteristics of the amphiphilic co-polymers via co-polymerization offers a facile method for controlling the bioavailability of anticancer drugs, ultimately increasing effectiveness and minimizing toxicity.
Keywords: AMPHIPHILIC BLOCK CO-POLYMER; CAMPTOTHECIN; CONTROLLED RELEASE; CRYSTALLINITY; CYCLIC KETAL; NANOPARTICLES.
Figures






Similar articles
-
Poly(ε-caprolactone)-based copolymers bearing pendant cyclic ketals and reactive acrylates for the fabrication of photocrosslinked elastomers.Acta Biomater. 2013 Sep;9(9):8232-44. doi: 10.1016/j.actbio.2013.06.005. Epub 2013 Jun 14. Acta Biomater. 2013. PMID: 23770222 Free PMC article.
-
Poly(ethyleneglycol)-b-poly(ε-caprolactone-co-γ-hydroxyl-ε- caprolactone) bearing pendant hydroxyl groups as nanocarriers for doxorubicin delivery.Biomacromolecules. 2012 Oct 8;13(10):3301-10. doi: 10.1021/bm301086c. Epub 2012 Sep 14. Biomacromolecules. 2012. PMID: 22931197
-
Facile synthesis of polyester-PEG triblock copolymers and preparation of amphiphilic nanoparticles as drug carriers.J Control Release. 2010 Dec 20;148(3):388-95. doi: 10.1016/j.jconrel.2010.09.017. Epub 2010 Sep 22. J Control Release. 2010. PMID: 20869413
-
Effect of structural factors on release profiles of camptothecin from block copolymer conjugates with high load of drug.Int J Pharm. 2018 Mar 1;538(1-2):231-242. doi: 10.1016/j.ijpharm.2018.01.022. Epub 2018 Jan 16. Int J Pharm. 2018. PMID: 29341920
-
Amorphous amphiphilic P(3HV-co-4HB)-b-mPEG block copolymer synthesized from bacterial copolyester via melt transesterification: nanoparticle preparation, cisplatin-loading for cancer therapy and in vitro evaluation.Eur J Pharm Biopharm. 2012 Apr;80(3):518-27. doi: 10.1016/j.ejpb.2011.11.014. Epub 2011 Dec 9. Eur J Pharm Biopharm. 2012. PMID: 22178562
Cited by
-
Dexamethasone-loaded block copolymer nanoparticles induce leukemia cell death and enhance therapeutic efficacy: a novel application in pediatric nanomedicine.Mol Pharm. 2013 Jun 3;10(6):2199-210. doi: 10.1021/mp300350e. Epub 2012 Nov 29. Mol Pharm. 2013. PMID: 23194373 Free PMC article.
-
Polyester-Based, Biodegradable Core-Multishell Nanocarriers for the Transport of Hydrophobic Drugs.Polymers (Basel). 2016 May 14;8(5):192. doi: 10.3390/polym8050192. Polymers (Basel). 2016. PMID: 30979288 Free PMC article.
-
Poly(ε-caprolactone)-based copolymers bearing pendant cyclic ketals and reactive acrylates for the fabrication of photocrosslinked elastomers.Acta Biomater. 2013 Sep;9(9):8232-44. doi: 10.1016/j.actbio.2013.06.005. Epub 2013 Jun 14. Acta Biomater. 2013. PMID: 23770222 Free PMC article.
-
A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics.Biomaterials. 2014 Mar;35(10):3319-30. doi: 10.1016/j.biomaterials.2013.12.080. Epub 2014 Jan 18. Biomaterials. 2014. PMID: 24447463 Free PMC article.
-
CD19-Targeted Nanodelivery of Doxorubicin Enhances Therapeutic Efficacy in B-Cell Acute Lymphoblastic Leukemia.Mol Pharm. 2015 Jun 1;12(6):2101-11. doi: 10.1021/acs.molpharmaceut.5b00071. Epub 2015 May 4. Mol Pharm. 2015. PMID: 25898125 Free PMC article.
References
-
- Panyam J, Labhasetwar V. Adv Drug Deliver Rev. 2003;55:329. - PubMed
-
- Allen TM, Cullis PR. Science. 2004;303:1818. - PubMed
-
- Rabinow BE. Nat Rev Drug Discov. 2004;3:785. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nat Rev Drug Discov. 2008;7:771. - PubMed
-
- Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. J Control Release. 2005;109:169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
