Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica
- PMID: 20594644
- PMCID: PMC2922436
- DOI: 10.1016/j.jhazmat.2010.06.019
Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica
Abstract
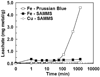
Copper(II) ferrocyanide on mesoporous silica (FC-Cu-EDA-SAMMS) has been evaluated against iron(III) hexacyanoferrate(II) (insoluble Prussian Blue) for removing cesium (Cs(+)) and thallium (Tl(+)) from natural waters and simulated acidic and alkaline wastes. From pH 0.1-7.3, FC-Cu-EDA-SAMMS had greater affinities for Cs and Tl and was less affected by the solution pH, competing cations, and matrices. SAMMS also outperformed Prussian Blue in terms of adsorption capacities (e.g., 21.7 versus 2.6 mg Cs/g in acidic waste stimulant (pH 1.1), 28.3 versus 5.8 mg Tl/g in seawater), and rate (e.g., over 95 wt% of Cs was removed from seawater after 2 min with SAMMS, while only 75 wt% was removed with Prussian Blue). SAMMS also had higher stability (e.g., 2.5-13-fold less Fe dissolved from 2 to 24 h of contact time). In addition to environmental applications, SAMMS has great potential to be used as orally administered drug for limiting the absorption of radioactive Cs and toxic Tl in gastrointestinal tract.
2010 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
In vitro and in vivo evaluation of a novel ferrocyanide functionalized nanopourous silica decorporation agent for cesium in rats.Health Phys. 2010 Sep;99(3):420-9. doi: 10.1097/HP.0b013e3181bca9b0. Health Phys. 2010. PMID: 20699707 Free PMC article.
-
Facile Synthesis of Prussian Blue Derivate-Modified Mesoporous Material via Photoinitiated Thiol-Ene Click Reaction for Cesium Adsorption.Chem Asian J. 2015 Aug;10(8):1738-44. doi: 10.1002/asia.201500350. Epub 2015 Jun 18. Chem Asian J. 2015. PMID: 25965318
-
Copper Ferrocyanide Functionalized Core-Shell Magnetic Silica Composites for the Selective Removal of Cesium Ions from Radioactive Liquid Waste.J Nanosci Nanotechnol. 2016 Jun;16(6):6223-30. doi: 10.1166/jnn.2016.10886. J Nanosci Nanotechnol. 2016. PMID: 27427694
-
Adsorption removal of cesium from drinking waters: a mini review on use of biosorbents and other adsorbents.Bioresour Technol. 2014 May;160:142-9. doi: 10.1016/j.biortech.2014.01.012. Epub 2014 Jan 11. Bioresour Technol. 2014. PMID: 24484852 Review.
-
Prussian Blue: A Safe Pigment with Zeolitic-Like Activity.Int J Mol Sci. 2021 Jan 15;22(2):780. doi: 10.3390/ijms22020780. Int J Mol Sci. 2021. PMID: 33467391 Free PMC article. Review.
Cited by
-
Molecular design of macrocyclic compounds for complete removal of thallium(I) from wastewater.Environ Sci Pollut Res Int. 2018 Dec;25(34):34550-34558. doi: 10.1007/s11356-018-3393-0. Epub 2018 Oct 12. Environ Sci Pollut Res Int. 2018. PMID: 30315528
-
Decontamination of radioactive cesium ions using ordered mesoporous monetite.RSC Adv. 2018 May 23;8(34):19041-19050. doi: 10.1039/c8ra02707b. eCollection 2018 May 22. RSC Adv. 2018. PMID: 35539644 Free PMC article.
-
In vitro and in vivo removal efficacy of insoluble Prussian blue in combination with calcium polystyrene sulfonate for thallium.Biometals. 2023 Oct;36(5):1125-1140. doi: 10.1007/s10534-023-00508-7. Epub 2023 May 24. Biometals. 2023. PMID: 37222858
-
Synthesis and Investigation of the Properties of Biphasic Hybrid Composites Based on Bentonite, Copper Hexacyanoferrate, Acrylamide and Acrylic Acid Hydrogel.Polymers (Basel). 2023 Jun 6;15(12):2586. doi: 10.3390/polym15122586. Polymers (Basel). 2023. PMID: 37376234 Free PMC article.
-
Nanoporous sorbent material as an oral phosphate binder and for aqueous phosphate, chromate, and arsenate removal.J Nanomed Nanotechnol. 2014;5:1000222. doi: 10.4172/2157-7439.1000222. J Nanomed Nanotechnol. 2014. PMID: 25554735 Free PMC article.
References
-
- Kubo AS, Rose DJ. Disposal of nuclear wastes: At an increased but still modest cost, more options can be explored and the outlook can be improved. Science. 1973;182:1205–1211. - PubMed
-
- Steefel CI, Carroll S, Zhao P, Roberts S. Cesium migration in Hanford sediment: a multisite cation exchange model based on laboratory transport experiments. J. Contam. Hydrol. 2003;67:219–246. - PubMed
-
- Gilmore WR. Radioactive waste disposal low and high level. New Jersey, USA: Noyes Data Corporation; 1977.
-
- Peterson S, Wymer RG. Chemistry in Nuclear Technology. USA: Addisson-Wesley Publishing; 1963.
-
- Peter AL, Viraraghavan T. Thallium: a review of public health and environmental concerns. Environ. Int. 2005;31:493–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

