Cu,Zn superoxide dismutase maturation and activity are regulated by COMMD1
- PMID: 20595380
- PMCID: PMC2937926
- DOI: 10.1074/jbc.M110.101477
Cu,Zn superoxide dismutase maturation and activity are regulated by COMMD1
Abstract
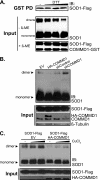
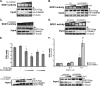
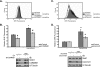
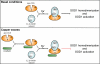
The maturation and activation of the anti-oxidant Cu,Zn superoxide dismutase (SOD1) are highly regulated processes that require several post-translational modifications. The maturation of SOD1 is initiated by incorporation of zinc and copper ions followed by disulfide oxidation leading to the formation of enzymatically active homodimers. Our present data indicate that homodimer formation is a regulated final step in SOD1 maturation and implicate the recently characterized copper homeostasis protein COMMD1 in this process. COMMD1 interacts with SOD1, and this interaction requires CCS-mediated copper incorporation into SOD1. COMMD1 does not regulate disulfide oxidation of SOD1 but reduces the level of SOD1 homodimers. RNAi-mediated knockdown of COMMD1 expression results in a significant induction of SOD1 activity and a consequent decrease in superoxide anion concentrations, whereas overexpression of COMMD1 exerts exactly the opposite effects. Here, we identify COMMD1 as a novel protein regulating SOD1 activation and associate COMMD1 function with the production of free radicals.
Figures




Similar articles
-
The Copper Metabolism MURR1 domain protein 1 (COMMD1) modulates the aggregation of misfolded protein species in a client-specific manner.PLoS One. 2014 Apr 1;9(4):e92408. doi: 10.1371/journal.pone.0092408. eCollection 2014. PLoS One. 2014. PMID: 24691167 Free PMC article.
-
Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase.Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2886-91. doi: 10.1073/pnas.040461197. Proc Natl Acad Sci U S A. 2000. PMID: 10694572 Free PMC article.
-
The yeast copper chaperone for copper-zinc superoxide dismutase (CCS1) is a multifunctional chaperone promoting all levels of SOD1 maturation.J Biol Chem. 2019 Feb 8;294(6):1956-1966. doi: 10.1074/jbc.RA118.005283. Epub 2018 Dec 10. J Biol Chem. 2019. PMID: 30530491 Free PMC article.
-
Oxygen-dependent activation of Cu,Zn-superoxide dismutase-1.Metallomics. 2017 Aug 16;9(8):1047-1059. doi: 10.1039/c6mt00298f. Metallomics. 2017. PMID: 28686251 Review.
-
Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space.Antioxid Redox Signal. 2010 Nov 1;13(9):1375-84. doi: 10.1089/ars.2010.3212. Antioxid Redox Signal. 2010. PMID: 20367259 Free PMC article. Review.
Cited by
-
Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic.Angew Chem Int Ed Engl. 2021 Apr 19;60(17):9215-9246. doi: 10.1002/anie.202000451. Epub 2020 Nov 19. Angew Chem Int Ed Engl. 2021. PMID: 32144830 Free PMC article. Review.
-
Charting the travels of copper in eukaryotes from yeast to mammals.Biochim Biophys Acta. 2012 Sep;1823(9):1580-93. doi: 10.1016/j.bbamcr.2012.02.011. Epub 2012 Feb 24. Biochim Biophys Acta. 2012. PMID: 22387373 Free PMC article. Review.
-
Functional diversity between HSP70 paralogs caused by variable interactions with specific co-chaperones.J Biol Chem. 2020 May 22;295(21):7301-7316. doi: 10.1074/jbc.RA119.012449. Epub 2020 Apr 13. J Biol Chem. 2020. PMID: 32284329 Free PMC article.
-
Commander Complex-A Multifaceted Operator in Intracellular Signaling and Cargo.Cells. 2021 Dec 7;10(12):3447. doi: 10.3390/cells10123447. Cells. 2021. PMID: 34943955 Free PMC article. Review.
-
The Copper Metabolism MURR1 domain protein 1 (COMMD1) modulates the aggregation of misfolded protein species in a client-specific manner.PLoS One. 2014 Apr 1;9(4):e92408. doi: 10.1371/journal.pone.0092408. eCollection 2014. PLoS One. 2014. PMID: 24691167 Free PMC article.
References
-
- Fridovich I. (1986) Adv. Enzymol. Relat. Areas Mol. Biol. 58, 61–97 - PubMed
-
- Goto J. J., Zhu H., Sanchez R. J., Nersissian A., Gralla E. B., Valentine J. S., Cabelli D. E. (2000) J. Biol. Chem. 275, 1007–1014 - PubMed
-
- Petrovic N., Comi A., Ettinger M. J. (1996) J. Biol. Chem. 271, 28331–28334 - PubMed
-
- Culotta V. C., Klomp L. W., Strain J., Casareno R. L., Krems B., Gitlin J. D. (1997) J. Biol. Chem. 272, 23469–23472 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

