HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42
- PMID: 20595387
- PMCID: PMC2937981
- DOI: 10.1074/jbc.M110.136770
HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42
Abstract
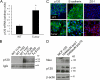
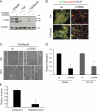
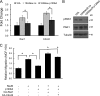
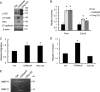
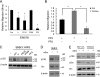
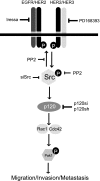
Breast cancers that overexpress the receptor tyrosine kinase ErbB2/HER2/Neu result in poor patient outcome because of extensive metastatic progression. Herein, we delineate a molecular mechanism that may govern this malignant phenotype. ErbB2 induction of migration requires activation of the small GTPases Rac1 and Cdc42. The ability of ErbB2 to activate these small GTPases necessitated expression of p120 catenin, which is itself up-regulated by signaling through ErbB2 and the tyrosine kinase Src. Silencing p120 in ErbB2-dependent breast cancer cell lines dramatically inhibited migration and invasion as well as activation of Rac1 and Cdc42. In contrast, overexpression of constitutively active mutants of these GTPases reversed the effects of p120 silencing. Lastly, ectopic expression of p120 promoted migration and invasion and potentiated metastatic progression of a weakly metastatic, ErbB2-dependent breast cancer cell line. These results suggest that p120 acts as an obligate intermediate between ErbB2 and Rac1/Cdc42 to modulate the metastatic potential of breast cancer cells.
Figures
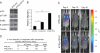

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

