Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities
- PMID: 20595624
- PMCID: PMC2950688
- DOI: 10.1152/ajpgi.00221.2010
Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities
Abstract
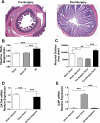
Surgical interposition of distal ileum into the proximal jejunum is a bariatric procedure that improves the metabolic syndrome. Changes in intestinal and hepatic physiology after ileal interposition (transposition) surgery (IIS) are not well understood. Our aim was to elucidate the adaptation of the interposed ileum, which we hypothesized, would lead to early bile acid reabsorption in the interposed ileum, thus short circuiting enterohepatic bile acid recycling to more proximal bowel segments. Rats with diet-induced obesity were randomized to IIS, with 10 cm of ileum repositioned distal to the duodenum, or sham surgery. A subgroup of sham rats was pair-fed to IIS rats. Physiological parameters were measured until 6 wk postsurgery. IIS rats ate less and lost more weight for the first 2 wk postsurgery. At study completion, body weights were not different, but IIS rats had reversed components of the metabolic syndrome. The interposed ileal segment adapted to a more jejunum-like villi length, mucosal surface area, and GATA4/ILBP mRNA. The interposed segment retained capacity for bile acid reabsorption and anorectic hormone secretion with the presence of ASBT and glucagon-like-peptide-1-positive cells in the villi. IIS rats had reduced primary bile acid levels in the proximal intestinal tract and higher primary bile acid levels in the serum, suggesting an early and efficient reabsorption of primary bile acids. IIS rats also had increased taurine and glycine-conjugated serum bile acids and reduced fecal bile acid loss. There was decreased hepatic Cyp27A1 mRNA with no changes in hepatic FXR, SHP, or NTCP expression. IIS protects against the metabolic syndrome through short-circuiting enterohepatic bile acid recycling. There is early reabsorption of primary bile acids despite selective "jejunization" of the interposed ileal segment. Changes in serum bile acids or bile acid enterohepatic recycling may mediate the metabolic benefits seen after bariatric surgery.
Figures





References
-
- Baker RD, Searle GW. Bile salt absorption at various levels of rat small intenstine. Proc Soc Exp Biol Med 105: 521–523, 1960 - PubMed
-
- Baxter JD, Webb P. Metabolism: bile acids heat things up. Nature 439: 402–403, 2006 - PubMed
-
- Boza C, Gagner M, Devaud N, Escalona A, Munoz R, Gandarillas M. Laparoscopic sleeve gastrectomy with ileal transposition (SGIT): A new surgical procedure as effective as gastric bypass for weight control in a porcine model. Surg Endosc 22: 1029–1034, 2008 - PubMed
-
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

