Proteasome inhibitors prevent caspase-1-mediated disease in rodents challenged with anthrax lethal toxin
- PMID: 20595632
- PMCID: PMC2913046
- DOI: 10.2353/ajpath.2010.090828
Proteasome inhibitors prevent caspase-1-mediated disease in rodents challenged with anthrax lethal toxin
Erratum in
- Am J Pathol. 2010 Oct;177(4):2145
Abstract
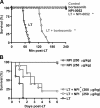
NOD-like receptors (NLRs) and caspase-1 are critical components of innate immunity, yet their over-activation has been linked to a long list of microbial and inflammatory diseases, including anthrax. The Bacillus anthracis lethal toxin (LT) has been shown to activate the NLR Nalp1b and caspase-1 and to induce many symptoms of the anthrax disease in susceptible murine strains. In this study we tested whether it is possible to prevent LT-mediated disease by pharmacological inhibition of caspase-1. We found that caspase-1 and proteasome inhibitors blocked LT-mediated caspase-1 activation and cytolysis of LT-sensitive (Fischer and Brown-Norway) rat macrophages. The proteasome inhibitor NPI-0052 also prevented disease progression and death in susceptible Fischer rats and increased survival in BALB/c mice after LT challenge. In addition, NPI-0052 blocked rapid disease progression and death in susceptible Fischer rats and BALB/c mice challenged with LT. In contrast, Lewis rats, which harbor LT-resistant macrophages, showed no signs of caspase-1 activation after LT injection and did not exhibit rapid disease progression. Taken together, our findings indicate that caspase-1 activation is critical for rapid disease progression in rodents challenged with LT. Our studies indicate that pharmacological inhibition of NLR signaling and caspase-1 can be used to treat inflammatory diseases.
Figures


Similar articles
-
Anthrax and the inflammasome.Microbes Infect. 2012 May;14(5):392-400. doi: 10.1016/j.micinf.2011.12.005. Epub 2011 Dec 17. Microbes Infect. 2012. PMID: 22207185 Free PMC article. Review.
-
Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing.J Biol Chem. 2007 Nov 23;282(47):34260-7. doi: 10.1074/jbc.M705687200. Epub 2007 Sep 17. J Biol Chem. 2007. PMID: 17878154
-
Killing of macrophages by anthrax lethal toxin: involvement of the N-end rule pathway.Cell Microbiol. 2008 Jun;10(6):1352-62. doi: 10.1111/j.1462-5822.2008.01131.x. Epub 2008 Feb 5. Cell Microbiol. 2008. PMID: 18266992 Free PMC article.
-
Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages.Infect Immun. 2009 Mar;77(3):1262-71. doi: 10.1128/IAI.01032-08. Epub 2009 Jan 5. Infect Immun. 2009. PMID: 19124602 Free PMC article.
-
New insights into the functions of anthrax toxin.Expert Rev Mol Med. 2006 Apr 11;8(7):1-18. doi: 10.1017/S1462399406010714. Expert Rev Mol Med. 2006. PMID: 16608555 Review.
Cited by
-
Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3.EMBO J. 2019 Feb 15;38(4):e99430. doi: 10.15252/embj.201899430. Epub 2019 Jan 7. EMBO J. 2019. PMID: 30617086 Free PMC article.
-
Distinct cathepsins control necrotic cell death mediated by pyroptosis inducers and lysosome-destabilizing agents.Cell Cycle. 2015;14(7):964-72. doi: 10.4161/15384101.2014.991194. Cell Cycle. 2015. PMID: 25830414 Free PMC article.
-
Anthrax and the inflammasome.Microbes Infect. 2012 May;14(5):392-400. doi: 10.1016/j.micinf.2011.12.005. Epub 2011 Dec 17. Microbes Infect. 2012. PMID: 22207185 Free PMC article. Review.
-
Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset.Oncoimmunology. 2015 May 27;4(9):e1034918. doi: 10.1080/2162402X.2015.1034918. eCollection 2015 Sep. Oncoimmunology. 2015. PMID: 26405600 Free PMC article.
-
Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death.Cell Cycle. 2013 Jun 15;12(12):1868-78. doi: 10.4161/cc.24903. Epub 2013 May 20. Cell Cycle. 2013. PMID: 23708522 Free PMC article.
References
-
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. - PubMed
-
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. - PubMed
-
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

