Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy
- PMID: 20595652
- PMCID: PMC2930621
- DOI: 10.1161/CIRCRESAHA.110.221481
Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy
Abstract
Rationale: The Purkinje fiber network has been proposed as the source of arrhythmogenic Ca(2+) release events in catecholaminergic polymorphic ventricular tachycardia (CPVT), yet evidence supporting this mechanism at the cellular level is lacking.
Objective: We sought to determine the frequency and severity of spontaneous Ca(2+) release events and the response to the antiarrhythmic agent flecainide in Purkinje cells and ventricular myocytes from RyR2(R4496C/+) CPVT mutant mice and littermate controls.
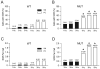
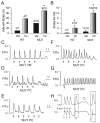
Methods and results: We crossed RyR2(R4496C/+) knock-in mice with the newly described Cntn2-EGFP BAC transgenic mice, which express a fluorescent reporter gene in cells of the cardiac conduction system, including the distal Purkinje fiber network. Isolated ventricular myocytes (EGFP(-)) and Purkinje cells (EGFP(+)) from wild-type hearts and mutant hearts were distinguished by epifluorescence and intracellular Ca(2+) dynamics recorded by microfluorimetry. Both wild-type and RyR2(R4496C/+) mutant Purkinje cells displayed significantly slower kinetics of activation and relaxation compared to ventricular myocytes of the same genotype, and tau(decay) in the mutant Purkinje cells was significantly slower than that observed in wild-type Purkinje cells. Of the 4 groups studied, RyR2(R4496C/+) mutant Purkinje cells were also most likely to develop spontaneous Ca(2+) release events, and the number of events per cell was also significantly greater. Furthermore, with isoproterenol treatment, although all 4 groups showed increases in the frequency of arrhythmogenic Ca(2+(i)) events, the RyR2(R4496C/+) Purkinje cells responded with the most profound abnormalities in intracellular Ca(2+) handling, including a significant increase in the frequency of unstimulated Ca(2+(i)) events and the development of alternans, as well as isolated and sustained runs of triggered beats. Both Purkinje cells and ventricular myocytes from wild-type mice showed suppression of spontaneous Ca(2+) release events with flecainide, whereas in RyR2(R4496C/+) mice, the Purkinje cells were preferentially responsive to drug. In contrast, the RyR2 blocker tetracaine was equally efficacious in mutant Purkinje cells and ventricular myocytes.
Conclusions: Purkinje cells display a greater propensity to develop abnormalities in intracellular Ca(2+) handling than ventricular myocytes. This proarrhythmic behavior is enhanced by disease-causing mutations in the RyR2 Ca(2+) release channel and greatly exacerbated by catecholaminergic stimulation, with the development of arrhythmogenic triggered beats. These data support the concept that Purkinje cells are critical contributors to arrhythmic triggers in animal models and humans with CPVT and suggest a broader role for the Purkinje fiber network in the genesis of ventricular arrhythmias.
Figures


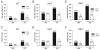
Similar articles
-
Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia.Heart Rhythm. 2010 Aug;7(8):1122-8. doi: 10.1016/j.hrthm.2010.06.010. Epub 2010 Jun 9. Heart Rhythm. 2010. PMID: 20538074 Free PMC article.
-
Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation.Cardiovasc Res. 2010 Jul 1;87(1):50-9. doi: 10.1093/cvr/cvq007. Epub 2010 Jan 15. Cardiovasc Res. 2010. PMID: 20080988
-
Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity.Circ Res. 2011 Jul 22;109(3):291-5. doi: 10.1161/CIRCRESAHA.111.247338. Epub 2011 Jun 16. Circ Res. 2011. PMID: 21680895
-
Inhibitors of Intracellular RyR2 Calcium Release Channels as Therapeutic Agents in Arrhythmogenic Heart Diseases.Annu Rev Pharmacol Toxicol. 2025 Jan;65(1):443-463. doi: 10.1146/annurev-pharmtox-061724-080739. Epub 2024 Dec 17. Annu Rev Pharmacol Toxicol. 2025. PMID: 39374431 Review.
-
Molecular, Subcellular, and Arrhythmogenic Mechanisms in Genetic RyR2 Disease.Biomolecules. 2022 Jul 26;12(8):1030. doi: 10.3390/biom12081030. Biomolecules. 2022. PMID: 35892340 Free PMC article. Review.
Cited by
-
Cardiac conduction system anomalies and sudden cardiac death: insights from murine models.Front Physiol. 2012 Jun 14;3:211. doi: 10.3389/fphys.2012.00211. eCollection 2012. Front Physiol. 2012. PMID: 22783196 Free PMC article.
-
The ionic bases of the action potential in isolated mouse cardiac Purkinje cell.Heart Rhythm. 2013 Jan;10(1):80-7. doi: 10.1016/j.hrthm.2012.10.002. Epub 2012 Oct 4. Heart Rhythm. 2013. PMID: 23041576 Free PMC article.
-
Gene mutations in cardiac arrhythmias: a review of recent evidence in ion channelopathies.Appl Clin Genet. 2013 Jan 18;6:1-13. doi: 10.2147/TACG.S29676. Print 2013. Appl Clin Genet. 2013. PMID: 23837003 Free PMC article.
-
Purkinje physiology and pathophysiology.J Interv Card Electrophysiol. 2018 Aug;52(3):255-262. doi: 10.1007/s10840-018-0414-3. Epub 2018 Jul 28. J Interv Card Electrophysiol. 2018. PMID: 30056516 Review.
-
Molecular Profiling of the Cardiac Conduction System: the Dawn of a New Era.Curr Cardiol Rep. 2021 Jul 1;23(8):103. doi: 10.1007/s11886-021-01536-w. Curr Cardiol Rep. 2021. PMID: 34196831 Review.
References
-
- Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009;6:1652–1659. - PubMed
-
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. - PubMed
-
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. - PMC - PubMed
-
- Myers DC, Fishman GI. Toward an understanding of the genetics of murine cardiac pacemaking and conduction system development. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:1018–1021. - PubMed
-
- Pennisi DJ, Rentschler S, Gourdie RG, Fishman GI, Mikawa T. Induction and patterning of the cardiac conduction system. Int J Dev Biol. 2002;46:765–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

