Soluble guanylate cyclase alpha1beta1 limits stroke size and attenuates neurological injury
- PMID: 20595671
- PMCID: PMC3047459
- DOI: 10.1161/STROKEAHA.109.577635
Soluble guanylate cyclase alpha1beta1 limits stroke size and attenuates neurological injury
Abstract
Background and purpose: Nitric oxide mediates endothelium-dependent vasodilation, modulates cerebral blood flow, and determines stroke outcome. Nitric oxide signals in part by stimulating soluble guanylate cyclase (sGC) to synthesize cGMP. To study the role of sGC in stroke injury, we compared the outcome of cerebral ischemia and reperfusion in mice deficient in the alpha(1) subunit of sGC (sGCalpha(1)(-/-)) with that in wild-type mice.
Methods: Blood pressure, cerebrovascular anatomy, and vasoreactivity of pressurized carotid arteries were compared in both mouse genotypes. Cerebral blood flow was measured before and during middle cerebral artery occlusion and reperfusion. We then assessed neurological deficit and infarct volume after 1 hour of occlusion and 23 hours of reperfusion and after 24 hours of occlusion.
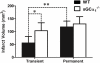
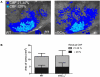
Results: Blood pressure and cerebrovascular anatomy were similar between genotypes. We found that vasodilation of carotid arteries in response to acetylcholine or sodium nitroprusside was diminished in sGCalpha(1)(-/-) compared with wild-type mice. Cerebral blood flow deficits did not differ between the genotypes during occlusion, but during reperfusion, cerebral blood flow was 45% less in sGCalpha(1)(-/-) mice. Infarct volumes and neurological deficits were similar after 24 hours of occlusion in both genotypes. After 1 hour of ischemia and 23 hours of reperfusion, infarct volumes were 2-fold larger and neurological deficits were worse in sGCalpha(1)(-/-) than in the wild-type mice.
Conclusions: sGCalpha(1) deficiency impairs vascular reactivity to nitric oxide and is associated with incomplete reperfusion, larger infarct size, and worse neurological damage, suggesting that cGMP generated by sGCalpha(1)beta(1) is protective in ischemic stroke.
Figures



References
-
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. - PMC - PubMed
-
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. - PubMed
-
- Willmot M, Gray L, Gibson C, Murphy S, Bath PM. A systematic review of nitric oxide donors and l-arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide. 2005;12:141–149. - PubMed
-
- Mergia E, Russwurm M, Zoidl G, Koesling D. Major occurrence of the new alpha(2)beta(1) isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal. 2003;15:189–195. - PubMed
-
- Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGC{alpha}1 knockout mice. Cardiovasc Res. 2008;79:179–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

