Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling
- PMID: 20595681
- PMCID: PMC2938592
- DOI: 10.1681/ASN.2009111153
Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling
Abstract
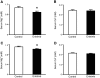
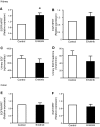
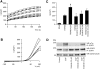
A mutation in pro-EGF causes isolated hypomagnesemia, and monoclonal antibodies targeting the extracellular domain of the EGF receptor (EGFR) affect epithelial Mg(2+) transport. The effect of the EGFR tyrosine kinase inhibitor erlotinib on Mg(2+) homeostasis, however, remains unknown. Here, we injected C57BL/6 mice with erlotinib for 23 days and observed a small but significant decrease in serum Mg(2+) concentrations at days 16 and 23, but the fractional excretion of Mg(2+) remained unchanged after 23 days. Semiquantitative immunohistochemical evaluation did not reveal detectable changes in renal expression of transient receptor potential melastatin 6 (TRPM6) protein, the channel that mediates Mg(2+) reabsorption. Patch clamp analysis in TRPM6-expressing cells demonstrated that 30 muM erlotinib inhibited EGF-induced changes in TRPM6 current density and tyrosine phosphorylation of EGFR; 0.3 muM erlotinib did not have these effects. Furthermore, 30 muM erlotinib inhibited EGF-stimulated increases in the mobile fraction of endomembrane TRPM6 channels. In summary, erlotinib can influence Mg(2+) handling but its effect on the systemic Mg(2+) concentration seems less potent than that observed with antibody-based EGFR inhibitors. These data suggest that typical human dosages of erlotinib are unlikely to severely affect serum Mg(2+) concentrations.
Figures
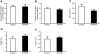

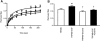
References
-
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M: Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002 - PubMed
-
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC: Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31: 171–174, 2002 - PubMed
-
- Groenestege WM, Hoenderop JG, van den Heuvel L, Knoers N, Bindels RJ: The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol 17: 1035–1043, 2006 - PubMed
-
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG: TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279: 19–25, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

