Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype
- PMID: 20595690
- PMCID: PMC2974386
- DOI: 10.2215/CJN.02210310
Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype
Abstract
Background and objectives: Hemolytic uremic syndrome (HUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment. Most childhood cases are caused by Shiga toxin-producing bacteria. The other form, atypical HUS (aHUS), accounts for 10% of cases and has a poor prognosis. Genetic complement abnormalities have been found in aHUS.
Design, setting, participants, and measurements: We screened 273 consecutive patients with aHUS for complement abnormalities and studied their role in predicting clinical phenotype and response to treatment. We compared mutation frequencies and localization and clinical outcome in familial (82) and sporadic (191) cases.
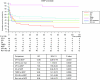
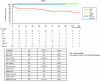
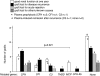
Results: In >70% of sporadic and familial cases, gene mutations, disease-associated factor H (CFH) polymorphisms, or anti-CFH autoantibodies were found. Either mutations or CFH polymorphisms were also found in the majority of patients with secondary aHUS, suggesting a genetic predisposition. Familial cases showed a higher prevalence of mutations in SCR20 of CFH and more severe disease than sporadic cases. Patients with CFH or THBD (thrombomodulin) mutations had the earliest onset and highest mortality. Membrane-cofactor protein (MCP) mutations were associated with the best prognosis. Plasma therapy induced remission in 55 to 80% of episodes in patients with CFH, C3, or THBD mutations or autoantibodies, whereas patients with CFI (factor I) mutations were poor responders. aHUS recurred frequently after kidney transplantation except for patients with MCP mutations.
Conclusions: Results underline the need of genetic screening for all susceptibility factors as part of clinical management of aHUS and for identification of patients who could safely benefit from kidney transplant.
Figures

References
-
- Ruggenenti P, Noris M, Remuzzi G: Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int 60: 831–846, 2001 - PubMed
-
- Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van de Kar N, Zimmerhackl LB: A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 70: 423–431, 2006 - PubMed
-
- Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 - PubMed
-
- Kavanagh D, Goodship TH: Update on evaluating complement in hemolytic uremic syndrome. Curr Opin Nephrol Hypertens 16: 565–571, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

