Molecular mechanisms of nutlin-induced apoptosis in multiple myeloma: evidence for p53-transcription-dependent and -independent pathways
- PMID: 20595817
- PMCID: PMC3040946
- DOI: 10.4161/cbt.10.6.12535
Molecular mechanisms of nutlin-induced apoptosis in multiple myeloma: evidence for p53-transcription-dependent and -independent pathways
Abstract
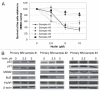
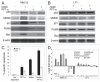
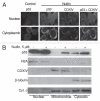
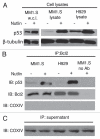
Multiple myeloma (MM) is an incurable plasma cell malignancy in which p53 is rarely mutated. Thus, activation of the p53 pathway by a small molecule inhibitor of the p53-MDM2 interaction, nutlin, in MM cells retaining wild type p53 is an attractive therapeutic strategy. Recently we reported that nutlin plus velcade (a proteasome inhibitor) displayed a synergistic response in MM. However, the mechanism of the p53-mediated apoptosis in MM has not been fully understood. Our data show that nutlin-induced apoptosis correlated with reduction in cell viability, upregulation of p53, p21 and MDM2 protein levels with a simultaneous increase in pro-apoptotic targets PUMA, Bax and Bak and downregulation of anti-apoptotic targets Bcl2 and survivin and activation of caspase in MM cells harboring wild type p53. Nutlin-induced apoptosis was inhibited when activation of caspase was blocked by the caspase inhibitor. Nutlin caused mitochondrial translocation of p53 where it binds with Bcl2, leading to cytochrome C release. Moreover, blocking the transcriptional arm of p53 by the p53-specific transcriptional inhibitor, pifithrin-α, not only inhibited nutlin-induced upregulation of p53-transcriptional targets but also augmented apoptosis in MM cells, suggesting an association of transcription-independent pathway of apoptosis. However, inhibitor of mitochondrial translocation of p53, PFT-μ, did not prevent nutlin-induced apoptosis, suggesting that the p53 transcription-dependent pathway was also operational in nutlin-induced apoptosis in MM. Our study provides the evidence that nutlin-induced apoptosis in MM cells is mediated by transcription-dependent and -independent pathways and supports further clinical evaluation of nutlin as a novel therapeutic agent in MM.
Figures




Comment in
-
Nutlin's two roads toward apoptosis.Cancer Biol Ther. 2010 Sep 15;10(6):579-81. doi: 10.4161/cbt.10.6.13127. Epub 2010 Sep 24. Cancer Biol Ther. 2010. PMID: 20716967 Free PMC article. No abstract available.
Similar articles
-
MDM2 antagonist nutlin plus proteasome inhibitor velcade combination displays a synergistic anti-myeloma activity.Cancer Biol Ther. 2010 Jun 1;9(11):936-44. doi: 10.4161/cbt.9.11.11882. Epub 2010 Jun 27. Cancer Biol Ther. 2010. PMID: 20418664
-
Activation of the p53 pathway by the MDM2 inhibitor nutlin-3a overcomes BCL2 overexpression in a preclinical model of diffuse large B-cell lymphoma associated with t(14;18)(q32;q21).Leukemia. 2011 May;25(5):856-67. doi: 10.1038/leu.2011.28. Epub 2011 Mar 11. Leukemia. 2011. PMID: 21394100 Free PMC article.
-
The transcription-independent mitochondrial p53 program is a major contributor to nutlin-induced apoptosis in tumor cells.Cell Cycle. 2009 Jun 1;8(11):1711-9. doi: 10.4161/cc.8.11.8596. Epub 2009 Jun 30. Cell Cycle. 2009. PMID: 19411846 Free PMC article.
-
Importance of proapoptotic protein PUMA in cell radioresistance.Folia Biol (Praha). 2014;60(2):53-6. doi: 10.14712/fb2014060020053. Folia Biol (Praha). 2014. PMID: 24785107 Review.
-
The ARTS of p53-dependent mitochondrial apoptosis.J Mol Cell Biol. 2023 Mar 29;14(10):mjac074. doi: 10.1093/jmcb/mjac074. J Mol Cell Biol. 2023. PMID: 36565718 Free PMC article. Review.
Cited by
-
c-Abl mediates angiotensin II-induced apoptosis in podocytes.J Mol Histol. 2013 Oct;44(5):597-608. doi: 10.1007/s10735-013-9505-8. Epub 2013 Mar 21. J Mol Histol. 2013. PMID: 23515840 Free PMC article.
-
Targeting the miR-221-222/PUMA/BAK/BAX Pathway Abrogates Dexamethasone Resistance in Multiple Myeloma.Cancer Res. 2015 Oct 15;75(20):4384-4397. doi: 10.1158/0008-5472.CAN-15-0457. Epub 2015 Aug 6. Cancer Res. 2015. PMID: 26249174 Free PMC article.
-
Pharmacologic activation of p53 by small-molecule MDM2 antagonists.Curr Pharm Des. 2011;17(6):560-8. doi: 10.2174/138161211795222603. Curr Pharm Des. 2011. PMID: 21391906 Free PMC article. Review.
-
p53 abnormalities and potential therapeutic targeting in multiple myeloma.Biomed Res Int. 2014;2014:717919. doi: 10.1155/2014/717919. Epub 2014 Jun 17. Biomed Res Int. 2014. PMID: 25028664 Free PMC article. Review.
-
Targeting the ubiquitin-mediated proteasome degradation of p53 for cancer therapy.Curr Pharm Des. 2013;19(18):3248-62. doi: 10.2174/1381612811319180009. Curr Pharm Des. 2013. PMID: 23151129 Free PMC article. Review.
References
-
- Fonseca R, Stewart KA. Targeted therapeutics for multiple myeloma. The arrival of a risk-stratified approach. Mol Cancer Ther. 2007;6:802–810. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Chang H, Qi C, Yi QL, Reece D, Stewart AK. p53 gene deletion detected by fluorescence in situ hybridization is an adverse prognostic factor for patients with multiple myeloma following autologous stem cell transplantation. Blood. 2005;105:358–360. - PubMed
-
- Chng WJ, Price-Troska T, Gonzalea-Paz N, Van Wier S, Jacobus S, Blood E, et al. Clinical significance of TP53 mutation in myeloma. Leukemia. 2007;13:582–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
