Glucocorticoids, stress, and fertility
- PMID: 20595939
- PMCID: PMC3547681
Glucocorticoids, stress, and fertility
Abstract
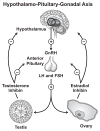
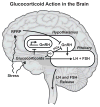
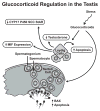
Modifications of the hypothalamo-pituitary-adrenal axis and associated changes in circulating levels of glucocorticoids form a key component of the response of an organism to stressful challenges. Increased levels of glucocorticoids promote gluconeogenesis, mobilization of amino acids, and stimulation of fat breakdown to maintain circulating levels of glucose necessary to mount a stress response. In addition to profound changes in the physiology and function of multiple tissues, stress and elevated glucocorticoids can also inhibit reproduction, a logical effect for the survival of self. Precise levels of glucocorticoids are required for proper gonadal function; where the balance is disrupted, so is fertility. Glucocorticoids affect gonadal function at multiple levels in hypothalamo-pituitary-gonadal axis: 1) the hypothalamus (to decrease the synthesis and release of gonadotropin-releasing hormone [GnRH]); 2) the pituitary gland (to inhibit the synthesis and release of luteinizing hormone [LH] and follicle stimulating hormone [FSH]); 3) the testis/ovary (to modulate steroidogenesis and/or gametogenesis directly). Furthermore, maternal exposure to prenatal stress or exogenous glucocorticoids can lead to permanent modification of hypothalamo-pituitary-adrenal function and stress-related behaviors in offspring. Glucocorticoids are vital to many aspects of normal brain development, but fetal exposure to superabundant glucocorticoids can result in life-long effects on neuroendocrine function. This review focuses on the molecular mechanisms believed to mediate glucocorticoid inhibition of reproductive functions and the anatomical sites at which these effects take place.
Figures

References
-
- Rabin D, et al. Stress and reproduction: physiologic and pathophysiologic interactions between the stress and reproductive axes. Adv Exp Med Biol. 1988;245:377–87. - PubMed
-
- Collu R, Gibb W, Ducharme JR. Effects of stress on the gonadal function. J Endocrinol Invest. 1984;7(5):529–37. - PubMed
-
- Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33(2):423–31. - PubMed
-
- Kamel F, Kubajak CL. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology. 1987;121(2):561–8. - PubMed
-
- Briski KP, Sylvester PW. Acute inhibition of pituitary LH release in the male rat by the glucocorticoid agonist decadron phosphate. Neuroendocrinology. 1991;54(4):313–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

