Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier
- PMID: 20595955
- PMCID: PMC3109429
- DOI: 10.1038/nprot.2010.76
Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier
Abstract
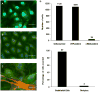
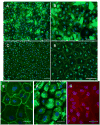
We describe a method for generating primary cultures of human brain microvascular endothelial cells (HBMVECs). HBMVECs are derived from microvessels isolated from temporal tissue removed during operative treatment of epilepsy. The tissue is mechanically fragmented and size filtered using polyester meshes. The resulting microvessel fragments are placed onto type I collagen-coated flasks to allow HBMVECs to migrate and proliferate. The overall process takes less than 3 h and does not require specialized equipment or enzymatic processes. HBMVECs are typically cultured for approximately 1 month until confluent. Cultures are highly pure ( approximately 97% endothelial cells; approximately 3% pericytes), are reproducible, and show characteristic brain endothelial markers (von Willebrand factor, glucose transporter-1) and robust expression of tight and adherens junction proteins as well as caveolin-1 and efflux protein P-glycoprotein. Monolayers of HBMVECs show characteristically high transendothelial electric resistance and have proven useful in multiple functional studies for in vitro modeling of the human blood-brain barrier.
Conflict of interest statement
Financial Interests
There are no competing financial interests by any of the authors.
Figures




References
-
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. - PubMed
-
- Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. - PubMed
-
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. - PubMed
-
- Ehrlich P. Das Sauerstoffbedürfnis des Organismus. Eine Farbenanalytische Studie; Hirschwald, Berlin: 1885.
-
- Joó F, Karnushina I. A procedure for the isolation of capillaries from rat brain. Cytobios. 1973;8:41–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

