Ciglitazone negatively regulates CXCL1 signaling through MITF to suppress melanoma growth
- PMID: 20596077
- PMCID: PMC3131866
- DOI: 10.1038/cdd.2010.75
Ciglitazone negatively regulates CXCL1 signaling through MITF to suppress melanoma growth
Abstract
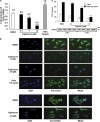
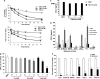
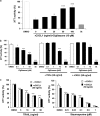
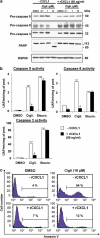
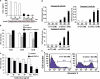
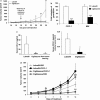
We have previously demonstrated that the thiazolidinedione ciglitazone inhibited, independently of PPARγ activation, melanoma cell growth. Further investigations now show that ciglitazone effects are mediated through the regulation of secreted factors. Q-PCR screening of several genes involved in melanoma biology reveals that ciglitazone inhibits expression of the CXCL1 chemokine gene. CXCL1 is overexpressed in melanoma and contributes to tumorigenicity. We show that ciglitazone induces a diminution of CXCL1 level in different human melanoma cell lines. This effect is mediated by the downregulation of microphthalmia-associated transcription factor, MITF, the master gene in melanocyte differentiation and involved in melanoma development. Further, recombinant CXCL1 protein is sufficient to abrogate thiazolidinedione effects such as apoptosis induction, whereas extinction of the CXCL1 pathway mimics phenotypic changes observed in response to ciglitazone. Finally, inhibition of human melanoma tumor development in nude mice treated with ciglitazone is associated with a strong decrease in MITF and CXCL1 levels. Our results show that anti-melanoma effects of thiazolidinediones involve an inhibition of the MITF/CXCL1 axis and highlight the key role of this specific pathway in melanoma malignancy.
Figures


References
-
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Demierre MF. Epidemiology and prevention of cutaneous melanoma. Curr Treat Options Oncol. 2006;7:181–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

