Alterations to proteins in the lens of hereditary Crygs-mutated cataractous mice
- PMID: 20596256
- PMCID: PMC2893057
Alterations to proteins in the lens of hereditary Crygs-mutated cataractous mice
Abstract
Purpose: To investigate the altered expression of proteins in the lens of mice with inherited cataracts.
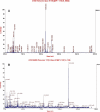
Methods: Mice with inherited cataracts caused by a spontaneous mutation of the gene gamma S-crystallin (Crygs) were used as the subjects. Lens proteins were extracted and separated by two-dimensional electrophoresis (2-DE). The spots representing differential proteins were first identified by image analysis, and then further analyzed by matrix assisted laser desorption/ionization-time of flight-tandem mass spectrometry (MALDI-TOF-MS/MS).
Results: 2-DE were conducted under high (882 microg) and low dosage (190 microg) of sample. Under each condition, the numbers of protein spots found in cataract lenses were similar to those in normal lenses (p>0.05). Seventeen proteins were identified in normal lenses, including alphaA- to alphaB-, betaA1- to betaA4-, betaB1- to betaB3-, gammaA- to gammaF-, and gammaS-crystallin, and bead-filament structure protein (BFSP/filensin). Seven differential ones were consistently identified. In the cataract lenses BFSP and gammaS-crystallin were absent; gammaF-crystallin was downregulated; and betaA1-, betaB1-, betaB2-, and alphaB-crystallin were upregulated. Those abnormally upregulated crystallins, when compared to normal ones, had smaller molecular weight, suggesting possible truncation.
Conclusions: The mutant Crygs gene can lead to changes of BFSP/filensin and other crystallins. The changes to these crystallins, together, may secondarily lead to cataract formation.
Figures


Similar articles
-
Proteomic analysis of water insoluble proteins from normal and cataractous human lenses.Mol Vis. 2007 Sep 14;13:1680-94. Mol Vis. 2007. PMID: 17893670
-
Susceptibility of ovine lens crystallins to proteolytic cleavage during formation of hereditary cataract.Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1016-22. doi: 10.1167/iovs.07-0792. Invest Ophthalmol Vis Sci. 2008. PMID: 18326725
-
Age-related changes in the water-soluble lens protein composition of Wistar and accelerated-senescence OXYS rats.Mol Vis. 2011;17:1457-67. Epub 2011 Jun 1. Mol Vis. 2011. PMID: 21677790 Free PMC article.
-
Genetics of crystallins: cataract and beyond.Exp Eye Res. 2009 Feb;88(2):173-89. doi: 10.1016/j.exer.2008.10.011. Epub 2008 Nov 1. Exp Eye Res. 2009. PMID: 19007775 Review.
-
Inherited cataracts: Genetic mechanisms and pathways new and old.Exp Eye Res. 2021 Aug;209:108662. doi: 10.1016/j.exer.2021.108662. Epub 2021 Jun 12. Exp Eye Res. 2021. PMID: 34126080 Free PMC article. Review.
References
-
- Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest Ophthalmol Vis Sci. 2002;43:216–24. - PubMed
-
- Ueda Y, Duncan MK, David LL. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci. 2002;43:205–15. - PubMed
-
- Li HW, Yao K, Jin HY, Sun LX, Lu DQ, Yu YB. Proteomic analysis of human lens epithelial cells exposed to microwaves. Jpn J Ophthalmol. 2007;51:412–6. - PubMed
-
- Tumminia SJ, Clark JI, Richiert DM, Mitton KP, Duglas-Tabor Y, Kowalak JA, Garland DL, Russell P. Three distinct stages of lens opacification in transgenic mice expressing the HIV-1 protease. Exp Eye Res. 2001;72:115–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
