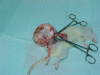
Breast tumor developed in a pregnant rat after treatment with the teratogen Cycloheximide
- PMID: 20596273
- PMCID: PMC2895279
Breast tumor developed in a pregnant rat after treatment with the teratogen Cycloheximide
Abstract
Aim: To describe histochemically and immunohistochemically a breast tumor presented in a pregnant rat given Cycloheximide to examine its teratogenic and embryotoxic effect on the embryos.
Methods: Cycloheximide was injected in pregnant Wistar rats at a dose of 3 mg/kg.b.w. on both 10th and 11th gestational days. In one of the rats, a large breast tumor developed rapidly. Histochemical staining with Hematoxylin-Eosin and Masson Trichrome and immunohistochemical identification with mouse monoclonal antibodies: a) Estrogen Receptor A and b) Estrogen Receptor B was performed.
Results: Analysis with A-receptor and B-receptor showed that the breast tumor which was developed after treatment with Cycloheximide was malignant. Positive immunohistochemical reaction was evident especially with A-receptor indicating the malignancy of the tumor.
Conclusions: Cycloheximide is a known toxic and teratogenic agent and potentially a carcinogenic drug. Thus it should be used with extreme caution as a pesticide.
Keywords: breast tumor; cycloheximide; estrogen receptors; teratogenesis; toxicity.
Figures

References
-
- Katyare SS, Fatterparker P, Sreenivasan A. Study of protein synthesis in rat liver mitochondria use of cycloheximide. Eur J Biochem. 1977;73:287–296. - PubMed
-
- Agrawal R, Pelkonen J, Mntyjrvi RA. Effect of Gamma-lnterferon or Cycloheximide Treatment on Viral and c-myc Transcripts in Bovine-Papillomavirus-Type-1-Transformed Primary Mouse Fibroblasts. Intervirology. 1993;36:44–52. - PubMed
-
- Mullauer L, Mosberger I, Grusch M, Rudas M, Chott A. Fas ligand is expressed in normal breast epithelial cells and is frequently up-regulated in breast cancer. Pathol. 2000;190:20–30. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
