Barium Titanate Nanoparticles: Highly Cytocompatible Dispersions in Glycol-chitosan and Doxorubicin Complexes for Cancer Therapy
- PMID: 20596329
- PMCID: PMC2894309
- DOI: 10.1007/s11671-010-9607-0
Barium Titanate Nanoparticles: Highly Cytocompatible Dispersions in Glycol-chitosan and Doxorubicin Complexes for Cancer Therapy
Abstract
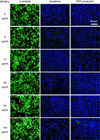
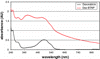
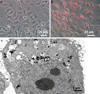
In the latest years, innovative nanomaterials have attracted a dramatic and exponentially increasing interest, in particular for their potential applications in the biomedical field. In this paper, we reported our findings on the cytocompatibility of barium titanate nanoparticles (BTNPs), an extremely interesting ceramic material. A rational and systematic study of BTNP cytocompatibility was performed, using a dispersion method based on a non-covalent binding to glycol-chitosan, which demonstrated the optimal cytocompatibility of this nanomaterial even at high concentration (100 μg/ml). Moreover, we showed that the efficiency of doxorubicin, a widely used chemotherapy drug, is highly enhanced following the complexation with BTNPs. Our results suggest that innovative ceramic nanomaterials such as BTNPs can be realistically exploited as alternative cellular nanovectors.
Keywords: Barium titanate nanoparticles; Cancer therapy; Doxorubicin; Glycol-chitosan; Neuroblastoma.
Figures



Similar articles
-
Antibody-Conjugated Barium Titanate Nanoparticles for Cell-Specific Targeting.ACS Appl Nano Mater. 2020 Mar 27;3(3):2636-2646. doi: 10.1021/acsanm.0c00019. Epub 2020 Mar 5. ACS Appl Nano Mater. 2020. PMID: 35873656 Free PMC article.
-
Design and development of poly-L/D-lactide copolymer and barium titanate nanoparticle 3D composite scaffolds using breath figure method for tissue engineering applications.Colloids Surf B Biointerfaces. 2021 Mar;199:111530. doi: 10.1016/j.colsurfb.2020.111530. Epub 2020 Dec 13. Colloids Surf B Biointerfaces. 2021. PMID: 33373840
-
Barium Titanate Nanoparticles Sensitise Treatment-Resistant Breast Cancer Cells to the Antitumor Action of Tumour-Treating Fields.Sci Rep. 2020 Feb 13;10(1):2560. doi: 10.1038/s41598-020-59445-x. Sci Rep. 2020. PMID: 32054945 Free PMC article.
-
A Comprehensive Review on Barium Titanate Nanoparticles as a Persuasive Piezoelectric Material for Biomedical Applications: Prospects and Challenges.Small. 2023 Mar;19(12):e2206401. doi: 10.1002/smll.202206401. Epub 2022 Dec 30. Small. 2023. PMID: 36585372 Review.
-
Functional Chitosan Nanoparticles in Cancer Treatment.J Biomed Nanotechnol. 2016 Aug;12(8):1585-603. doi: 10.1166/jbn.2016.2228. J Biomed Nanotechnol. 2016. PMID: 29341581 Review.
Cited by
-
Size-dependent ecotoxicity of barium titanate particles: the case of Chlorella vulgaris green algae.Ecotoxicology. 2015 May;24(4):938-48. doi: 10.1007/s10646-015-1436-6. Epub 2015 Mar 13. Ecotoxicology. 2015. PMID: 25763523
-
Neuron Compatibility and Antioxidant Activity of Barium Titanate and Lithium Niobate Nanoparticles.Int J Mol Sci. 2022 Feb 3;23(3):1761. doi: 10.3390/ijms23031761. Int J Mol Sci. 2022. PMID: 35163681 Free PMC article.
-
Folate-conjugated boron nitride nanospheres for targeted delivery of anticancer drugs.Int J Nanomedicine. 2016 Sep 12;11:4573-4582. doi: 10.2147/IJN.S110689. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27695318 Free PMC article.
-
Micropatterned Styrene-Butadiene-Styrene Thin Films Doped with Barium Titanate Nanoparticles: Effects on Myoblast Differentiation.ACS Biomater Sci Eng. 2025 May 12;11(5):2910-2921. doi: 10.1021/acsbiomaterials.4c02468. Epub 2025 May 1. ACS Biomater Sci Eng. 2025. PMID: 40309959 Free PMC article.
-
Comparison of Methods for Surface Modification of Barium Titanate Nanoparticles for Aqueous Dispersibility: Toward Biomedical Utilization of Perovskite Oxides.ACS Appl Mater Interfaces. 2020 Nov 18;12(46):51135-51147. doi: 10.1021/acsami.0c10063. Epub 2020 Nov 3. ACS Appl Mater Interfaces. 2020. PMID: 32988209 Free PMC article.
References
-
- Koo OM, Rubinstein I, Onyuksel H. Nanomedicine: NBM. 2005. p. 193. COI number [1:CAS:528:DC%2BD2MXht1Cgt7nI] - PubMed
-
- Amiji MM. Nanomedicine: NBM. 2006. p. 299.
-
- Wood J. In: The cytotoxic Handbook. Allwood M, Stanley A, Wright P, editor. Radcliffe Medical Press Ltd, Oxon, UK; 2002. Doxorubicin; pp. 322–329.
-
- Liao LB, Zhou HY, Xiao XM. J. 2005. p. 108. COI number [1:CAS:528:DC%2BD2MXls1ygu7Y%3D]; Bibcode number [2005JMoSt.749..108L] - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

