Assessment of the In Vivo Toxicity of Gold Nanoparticles
- PMID: 20596373
- PMCID: PMC2894102
- DOI: 10.1007/s11671-009-9334-6
Assessment of the In Vivo Toxicity of Gold Nanoparticles
Abstract
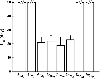
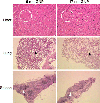
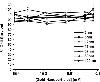
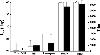
The environmental impact of nanoparticles is evident; however, their toxicity due to their nanosize is rarely discussed. Gold nanoparticles (GNPs) may serve as a promising model to address the size-dependent biological response to nanoparticles because they show good biocompatibility and their size can be controlled with great precision during their chemical synthesis. Naked GNPs ranging from 3 to 100 nm were injected intraperitoneally into BALB/C mice at a dose of 8 mg/kg/week. GNPs of 3, 5, 50, and 100 nm did not show harmful effects; however, GNPs ranging from 8 to 37 nm induced severe sickness in mice. Mice injected with GNPs in this range showed fatigue, loss of appetite, change of fur color, and weight loss. Starting from day 14, mice in this group exhibited a camel-like back and crooked spine. The majority of mice in these groups died within 21 days. Injection of 5 and 3 nm GNPs, however, did not induce sickness or lethality in mice. Pathological examination of the major organs of the mice in the diseased groups indicated an increase of Kupffer cells in the liver, loss of structural integrity in the lungs, and diffusion of white pulp in the spleen. The pathological abnormality was associated with the presence of gold particles at the diseased sites, which were verified by ex vivo Coherent anti-Stoke Raman scattering microscopy. Modifying the surface of the GNPs by incorporating immunogenic peptides ameliorated their toxicity. This reduction in the toxicity is associated with an increase in the ability to induce antibody response. The toxicity of GNPs may be a fundamental determinant of the environmental toxicity of nanoparticles.
Figures



Similar articles
-
Size-dependent impairment of cognition in mice caused by the injection of gold nanoparticles.Nanotechnology. 2010 Dec 3;21(48):485102. doi: 10.1088/0957-4484/21/48/485102. Epub 2010 Nov 4. Nanotechnology. 2010. PMID: 21051801
-
Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide.Nanotechnology. 2010 May 14;21(19):195101. doi: 10.1088/0957-4484/21/19/195101. Epub 2010 Apr 19. Nanotechnology. 2010. PMID: 20400818
-
Gold nanoparticles regulate the blimp1/pax5 pathway and enhance antibody secretion in B-cells.Nanotechnology. 2014 Mar 28;25(12):125103. doi: 10.1088/0957-4484/25/12/125103. Epub 2014 Feb 27. Nanotechnology. 2014. PMID: 24576992
-
Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles.Molecules. 2018 Jul 25;23(8):1848. doi: 10.3390/molecules23081848. Molecules. 2018. PMID: 30044410 Free PMC article.
-
Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia.Lipids Health Dis. 2011 Aug 5;10:133. doi: 10.1186/1476-511X-10-133. Lipids Health Dis. 2011. PMID: 21819574 Free PMC article.
Cited by
-
Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics.Front Chem. 2021 Feb 23;9:629635. doi: 10.3389/fchem.2021.629635. eCollection 2021. Front Chem. 2021. PMID: 33708759 Free PMC article. Review.
-
In vitro perforation of human epithelial carcinoma cell with antibody-conjugated biodegradable microspheres illuminated by a single 80 femtosecond near-infrared laser pulse.Int J Nanomedicine. 2012;7:2653-60. doi: 10.2147/IJN.S31768. Epub 2012 May 28. Int J Nanomedicine. 2012. PMID: 22679375 Free PMC article.
-
Bio-Synthesis of Aspergillus terreus Mediated Gold Nanoparticle: Antimicrobial, Antioxidant, Antifungal and In Vitro Cytotoxicity Studies.Materials (Basel). 2022 May 29;15(11):3877. doi: 10.3390/ma15113877. Materials (Basel). 2022. PMID: 35683175 Free PMC article.
-
Treasure on the Earth-Gold Nanoparticles and Their Biomedical Applications.Materials (Basel). 2022 May 7;15(9):3355. doi: 10.3390/ma15093355. Materials (Basel). 2022. PMID: 35591689 Free PMC article. Review.
-
The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment.Int J Mol Sci. 2020 Apr 3;21(7):2480. doi: 10.3390/ijms21072480. Int J Mol Sci. 2020. PMID: 32260051 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

