Nanoparticles of Poly(Lactide-Co-Glycolide)-d-a-Tocopheryl Polyethylene Glycol 1000 Succinate Random Copolymer for Cancer Treatment
- PMID: 20596457
- PMCID: PMC2893931
- DOI: 10.1007/s11671-010-9620-3
Nanoparticles of Poly(Lactide-Co-Glycolide)-d-a-Tocopheryl Polyethylene Glycol 1000 Succinate Random Copolymer for Cancer Treatment
Abstract
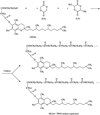
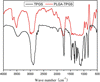
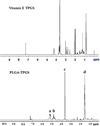
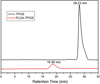
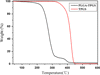
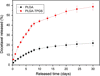
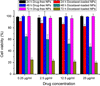
Cancer is the leading cause of death worldwide. Nanomaterials and nanotechnologies could provide potential solutions. In this research, a novel biodegradable poly(lactide-co-glycolide)-d-a-tocopheryl polyethylene glycol 1000 succinate (PLGA-TPGS) random copolymer was synthesized from lactide, glycolide and d-a-tocopheryl polyethylene glycol 1000 succinate (TPGS) by ring-opening polymerization using stannous octoate as catalyst. The obtained random copolymers were characterized by 1H NMR, FTIR, GPC and TGA. The docetaxel-loaded nanoparticles made of PLGA-TPGS copolymer were prepared by a modified solvent extraction/evaporation method. The nanoparticles were then characterized by various state-of-the-art techniques. The results revealed that the size of PLGA-TPGS nanoparticles was around 250 nm. The docetaxel-loaded PLGA-TPGS nanoparticles could achieve much faster drug release in comparison with PLGA nanoparticles. In vitro cellular uptakes of such nanoparticles were investigated by CLSM, demonstrating the fluorescence PLGA-TPGS nanoparticles could be internalized by human cervix carcinoma cells (HeLa). The results also indicated that PLGA-TPGS-based nanoparticles were biocompatible, and the docetaxel-loaded PLGA-TPGS nanoparticles had significant cytotoxicity against Hela cells. The cytotoxicity against HeLa cells for PLGA-TPGS nanoparticles was in time- and concentration-dependent manner. In conclusion, PLGA-TPGS random copolymer could be acted as a novel and promising biocompatible polymeric matrix material applicable to nanoparticle-based drug delivery system for cancer chemotherapy.
Keywords: Cancer chemotherapy; Docetaxel; HeLa; Nanoparticle; PLGA-TPGS; Random copolymer.
Figures



References
-
- Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine. 2005;1:101–109. COI number [1:CAS:528:DC%2BD2MXpsFCgtrc%3D] - PubMed
-
- Kim KY. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine. 2007;3:103–110. COI number [1:CAS:528:DC%2BD2sXnsVOrtLo%3D] - PubMed
-
- Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomedicine. 2007;3:20–31. COI number [1:CAS:528:DC%2BD2sXktFCmsrk%3D] - PubMed
-
- Zhou S, Xu J, Yang H, Deng X. Synthesis and characterization of biodegradable poly(ε-caprolactone)-polyglycolide-poly(ethylene glycol) monomethyl ether random copolymer. Macromol. 2004;289:576–580. doi: 10.1002/mame.200300283. COI number [1:CAS:528:DC%2BD2cXlsFGrt7c%3D] - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

