Molecular composition of staufen2-containing ribonucleoproteins in embryonic rat brain
- PMID: 20596529
- PMCID: PMC2893162
- DOI: 10.1371/journal.pone.0011350
Molecular composition of staufen2-containing ribonucleoproteins in embryonic rat brain
Abstract
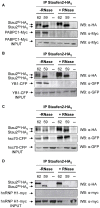
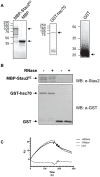
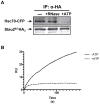
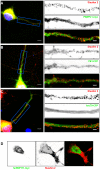
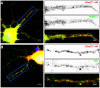
Messenger ribonucleoprotein particles (mRNPs) are used to transport mRNAs along neuronal dendrites to their site of translation. Numerous mRNA-binding and regulatory proteins within mRNPs finely regulate the fate of bound-mRNAs. Their specific combination defines different types of mRNPs that in turn are related to specific synaptic functions. One of these mRNA-binding proteins, Staufen2 (Stau2), was shown to transport dendritic mRNAs along microtubules. Its knockdown expression in neurons was shown to change spine morphology and synaptic functions. To further understand the molecular mechanisms by which Stau2 modulates synaptic function in neurons, it is important to identify and characterize protein co-factors that regulate the fate of Stau2-containing mRNPs. To this end, a proteomic approach was used to identify co-immunoprecipitated proteins in Staufen2-containing mRNPs isolated from embryonic rat brains. The proteomic approach identified mRNA-binding proteins (PABPC1, hnRNP H1, YB1 and hsc70), proteins of the cytoskeleton (alpha- and beta-tubulin) and RUFY3 a poorly characterized protein. While PABPC1 and YB1 associate with Stau2-containing mRNPs through RNAs, hsc70 is directly bound to Stau2 and this interaction is regulated by ATP. PABPC1 and YB1 proteins formed puncta in dendrites of embryonic rat hippocampal neurons. However, they poorly co-localized with Stau2 in the large dendritic complexes suggesting that they are rather components of Stau2-containing mRNA particles. All together, these results represent a further step in the characterization of Stau2-containing mRNPs in neurons and provide new tools to study and understand how Stau2-containing mRNPs are transported, translationally silenced during transport and/or locally expressed according to cell needs.
Conflict of interest statement
Figures
Similar articles
-
A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes.RNA. 2008 Feb;14(2):324-35. doi: 10.1261/rna.720308. Epub 2007 Dec 19. RNA. 2008. PMID: 18094122 Free PMC article.
-
Genome wide identification of Staufen2-bound mRNAs in embryonic rat brains.BMB Rep. 2010 May;43(5):344-8. doi: 10.5483/bmbrep.2010.43.5.344. BMB Rep. 2010. PMID: 20510018
-
The brain-specific double-stranded RNA-binding protein Staufen2 is required for dendritic spine morphogenesis.J Cell Biol. 2006 Jan 16;172(2):221-31. doi: 10.1083/jcb.200509035. J Cell Biol. 2006. PMID: 16418534 Free PMC article.
-
The role of mammalian Staufen on mRNA traffic: a view from its nucleocytoplasmic shuttling function.Cell Struct Funct. 2005;30(2):51-6. doi: 10.1247/csf.30.51. Cell Struct Funct. 2005. PMID: 16377940 Review.
-
Cataloguing and Selection of mRNAs Localized to Dendrites in Neurons and Regulated by RNA-Binding Proteins in RNA Granules.Biomolecules. 2020 Jan 22;10(2):167. doi: 10.3390/biom10020167. Biomolecules. 2020. PMID: 31978946 Free PMC article. Review.
Cited by
-
The RUFYs, a Family of Effector Proteins Involved in Intracellular Trafficking and Cytoskeleton Dynamics.Front Cell Dev Biol. 2020 Aug 11;8:779. doi: 10.3389/fcell.2020.00779. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850870 Free PMC article. Review.
-
Genomic analysis of RNA localization.RNA Biol. 2014;11(8):1040-50. doi: 10.4161/rna.32146. RNA Biol. 2014. PMID: 25483039 Free PMC article. Review.
-
Intracellular and intercellular transport of RNA organelles in CXG repeat disorders: The strength of weak ties.Front Mol Biosci. 2022 Dec 16;9:1000932. doi: 10.3389/fmolb.2022.1000932. eCollection 2022. Front Mol Biosci. 2022. PMID: 36589236 Free PMC article. Review.
-
Translational co-regulation of a ligand and inhibitor by a conserved RNA element.Nucleic Acids Res. 2018 Jan 9;46(1):104-119. doi: 10.1093/nar/gkx938. Nucleic Acids Res. 2018. PMID: 29059375 Free PMC article.
-
Quantitative STAU2 measurement in lymphocytes for breast cancer risk assessment.Sci Rep. 2021 Jan 13;11(1):915. doi: 10.1038/s41598-020-79622-2. Sci Rep. 2021. PMID: 33441653 Free PMC article.
References
-
- Bassell GJ, Oleynikov Y, Singer RH. The travels of mRNAs through all cells large and small. FASEB Journal. 1999;13:447–454. - PubMed
-
- Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. - PubMed
-
- Sossin WS, DesGroseillers L. Intracellular trafficking of RNA in neurons. Traffic. 2006;7:1581–1589. - PubMed
-
- Sanchez-Carbente M, DesGroseillers L. Understanding the importance of mRNA transport in memory. Prog Brain Res. 2008;169:41–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

