Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration
- PMID: 20597071
- PMCID: PMC3246453
- DOI: 10.1002/hep.23779
Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration
Abstract
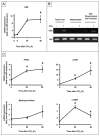
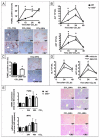
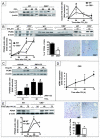
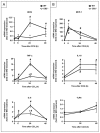
The cannabinoid receptor 2 (CB2) plays a pleiotropic role in innate immunity and is a crucial mediator of liver disease. In this study, we investigated the impact of CB2 receptors on the regenerative process associated with liver injury. Following acute hepatitis induced by carbon tetrachloride (CCl(4)), CB2 was induced in the nonparenchymal cell fraction and remained undetectable in hepatocytes. Administration of CCl(4) to CB2(-/-) mice accelerated liver injury, as shown by increased alanine/aspartate aminotransferase levels and hepatocyte apoptosis, and delayed liver regeneration, as reflected by a retarded induction of hepatocyte proliferating cell nuclear antigen expression; proliferating cell nuclear antigen induction was also delayed in CB2(-/-) mice undergoing partial hepatectomy. Conversely, following treatment with the CB2 agonist JWH-133, CCl(4)-treated WT mice displayed reduced liver injury and accelerated liver regeneration. The CCl(4)-treated CB2(-/-) mice showed a decrease in inducible nitric oxide synthase and tumor necrosis factor-alpha expression, and administration of the nitric oxide donor moldomine (SIN-1) to these animals reduced hepatocyte apoptosis, without affecting liver regeneration. Impaired liver regeneration was consecutive to an interleukin-6 (IL-6)-mediated decrease in matrix metalloproteinase 2 (MMP-2) activity. Indeed, CCl(4)-treated CB2(-/-) mice displayed lower levels of hepatic IL-6 messenger RNA and increased MMP-2 activity. Administration of IL-6 to these mice decreased MMP-2 activity and improved liver regeneration, without affecting hepatocyte apoptosis. Accordingly, administration of the MMP inhibitor CTTHWGFTLC to CCl(4)-treated CB2(-/-) mice improved liver regeneration. Finally, in vitro studies demonstrated that incubation of hepatic myofibroblasts with JWH-133 increased tumor necrosis factor-alpha and IL-6 and decreased MMP-2 expressions.
Conclusion: CB2 receptors reduce liver injury and promote liver regeneration following acute insult, via distinct paracrine mechanisms involving hepatic myofibroblasts. These results suggest that CB2 agonists display potent hepatoprotective properties, in addition to their antifibrogenic effects.
Figures
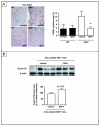
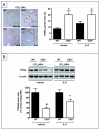

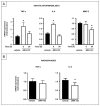
Comment in
-
Association of the cannabinoid receptor 2 (CB2) Gln63Arg polymorphism with indices of liver damage in obese children: an alternative way to highlight the CB2 hepatoprotective properties.Hepatology. 2011 Sep 2;54(3):1102; author reply 1102-3. doi: 10.1002/hep.24440. Epub 2011 Jul 21. Hepatology. 2011. PMID: 21608006 No abstract available.
Similar articles
-
Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury.J Gastroenterol Hepatol. 2014 Mar;29(3):603-13. doi: 10.1111/jgh.12366. J Gastroenterol Hepatol. 2014. PMID: 23981054
-
Hepatoprotective effects of baicalein against CCl₄-induced acute liver injury in mice.World J Gastroenterol. 2012 Dec 7;18(45):6605-13. doi: 10.3748/wjg.v18.i45.6605. World J Gastroenterol. 2012. PMID: 23236235 Free PMC article.
-
Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice.Hepatology. 2011 Oct;54(4):1217-26. doi: 10.1002/hep.24524. Epub 2011 Sep 6. Hepatology. 2011. PMID: 21735467
-
Effect of Hepatic Pathology on Liver Regeneration: The Main Metabolic Mechanisms Causing Impaired Hepatic Regeneration.Int J Mol Sci. 2023 May 23;24(11):9112. doi: 10.3390/ijms24119112. Int J Mol Sci. 2023. PMID: 37298064 Free PMC article. Review.
-
Key hepatoprotective roles of mitochondria in liver regeneration.Am J Physiol Gastrointest Liver Physiol. 2023 Mar 1;324(3):G207-G218. doi: 10.1152/ajpgi.00220.2022. Epub 2023 Jan 17. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 36648139 Free PMC article. Review.
Cited by
-
Role of the sympathetic nervous system in carbon tetrachloride-induced hepatotoxicity and systemic inflammation.PLoS One. 2015 Mar 23;10(3):e0121365. doi: 10.1371/journal.pone.0121365. eCollection 2015. PLoS One. 2015. PMID: 25799095 Free PMC article.
-
β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice.Br J Pharmacol. 2018 Jan;175(2):320-334. doi: 10.1111/bph.13722. Epub 2017 Feb 22. Br J Pharmacol. 2018. PMID: 28107775 Free PMC article.
-
Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs.J Pharmacol Exp Ther. 2015 May;353(2):369-79. doi: 10.1124/jpet.114.220368. Epub 2015 Mar 6. J Pharmacol Exp Ther. 2015. PMID: 25749929 Free PMC article.
-
The role of endocannabinoids system in fatty liver disease and therapeutic potentials.Saudi J Gastroenterol. 2013 Jul-Aug;19(4):144-51. doi: 10.4103/1319-3767.114505. Saudi J Gastroenterol. 2013. PMID: 23828743 Free PMC article. Review.
-
The cellular pathways of liver fibrosis in non-alcoholic steatohepatitis.Ann Transl Med. 2020 Mar;8(6):400. doi: 10.21037/atm.2020.02.184. Ann Transl Med. 2020. PMID: 32355844 Free PMC article. Review.
References
-
- Mallat A, Lotersztajn S. Endocannabinoids and Their Receptors in the Liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G9–G12. - PubMed
-
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. - PubMed
-
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature Medicine. 2005;434:782–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
